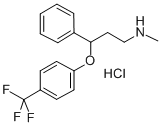
Atomoxetine hydrochloride
Synonym(s):(R)-N-Methyl-γ-(2-methyl-phenoxy)benzenepropanamine hydrochloride;(R)-Tomoxetine hydrochloride;Atomoxetine hydrochloride
- CAS NO.:82248-59-7
- Empirical Formula: C17H22ClNO
- Molecular Weight: 291.82
- MDL number: MFCD06410992
- EINECS: 629-925-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
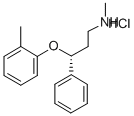
What is Atomoxetine hydrochloride?
Description
Atomoxetine is the first non-stimulant marketed for the treatment of attention deficit hyperactivity disorder (ADHD). It is the R-stereoisomer of the racemate tomoxetine and is a selective and potent norepinephrine uptake inhibitor (Ki=0.7–1.9 nM) that is devoid of binding to monoamine receptor. It also has little effect on dopamine and serotonin reuptake or acetylcholine, H1 histamine, alpha1 or alpha1-adrenergic or dopamine receptors. It is prepared from racemic 1-phenylbut-3-en-1-ol via a selective enzymatic acylation leaving the desired S-stereoisomer as the alcohol. This alcohol is converted via a Mitsunobu reaction with ortho-cresol to the corresponding ether with isomeric R-configuration. Ozonolysis and reduction steps provided the terminal alcohol that is mesylated and displaced with methylamine. Its selectivity for norepinephrine relative to dopamine inhibition was demonstrated in vivo preclinically. In a two-lever (two condition) discriminative stimulus effect study in squirrel monkeys, tomoxetine and other norepinephrine uptake inhibitors substituted for cocaine under low-dose training conditions, whereas dopamine uptake inhibitors substituted for cocaine in both low and high-dose conditions. In clinical ADHD studies in adolescents, it was significantly different from placebo in 1.2 and 1.8 mpk/day dosing. In the clinical study in adults using the CAARS scale a 95 mg/day dose provided greater than 30% improvement in total scores. Atomoxetine is about 63% orally bioavailable, is highly protein bound (98%, primarily to albumin) and has a half-life of about 5.2 h. It is metabolized by CYP2D6 resulting in differential clearance for poor metabolizers (halflife of 19 h with a 10 times higher AUC) relative to extensive metabolizers. The total daily dose for children, adolescents and adults is a maximum of 100 mg/day. Common side effects in children and adults include nausea, decreased appetite, and dizziness. Adults may also have insomnia.
Description
Atomoxetine is a selective norepinephrine reuptake inhibitor with Ki values of 5, 77, and 1,451 nM for norepinephrine, serotonin, and dopamine transporters, respectively. It is selective over the choline, GABA, and adenosine transporters, and a number of neurotransmitter receptors, ion channels, second messengers, and brain/gut peptides. In the rat prefrontal cortex (PFC), it increases extracellular norepinephrine and dopamine by 3-fold and increases Fos expression. Atomoxetine (0.1, 0.5, and 1 mg/kg) reduces premature responding, a measure of impulsivity, by rats in the 5-choice serial reaction time test (5CSRTT) in a dose-dependent manner. It also has neuroprotective effects when administered prior to ischemic damage in a gerbil model of transient cerebral ischemia. Formulations containing atomoxetine have been used in the treatment of attention-deficit hyperactivity disorder (ADHD) in children, adolescents, and adults.
Chemical properties
White Solid
Originator
Eli Lilly & Co (US)
The Uses of Atomoxetine hydrochloride
(R)-Tomoxetine hydrochloride has been used as a noradrenaline reuptake inhibitor:
- to study the role of L-threo-3,4-dihydroxyphenylserine (L-DOPS) in the pathogenesis of Alzheimer′s disease in mice
- to study its effects on set shifting in rats
- to study its effects on rat brain as a result of its long-term use
The Uses of Atomoxetine hydrochloride
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Atomoxetine hydrochloride may be used as a pharmaceutical reference standard for the determination of atomoxetine hydrochloride in pharmaceutical formulations by spectrophotometric method.
The Uses of Atomoxetine hydrochloride
Psychotherapeutic, Anti Depressant
The Uses of Atomoxetine hydrochloride
A Norepinephrine reuptake inhibitor
What are the applications of Application
Tomoxetine hydrochloride is a selective SLC6A2 inhibitor
Definition
ChEBI: The hydrochloride salt of atomoxetine.
brand name
Strattera (Lilly).
General Description
Atomoxetine hydrochloride is a nonstimulant used in the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) in children and adults.
Biological Activity
Potent and selective noradrenalin re-uptake inhibitor (K i values are 5, 77 and 1451 nM for inhibition of radioligand binding to human NET, SERT and DAT respectively). Displays minimal affinity for a range of other neurotransmitter receptors and transporters (K i > 1 μ M). Antidepressant.
Biochem/physiol Actions
Norepinephrine uptake blocker.
Synthesis
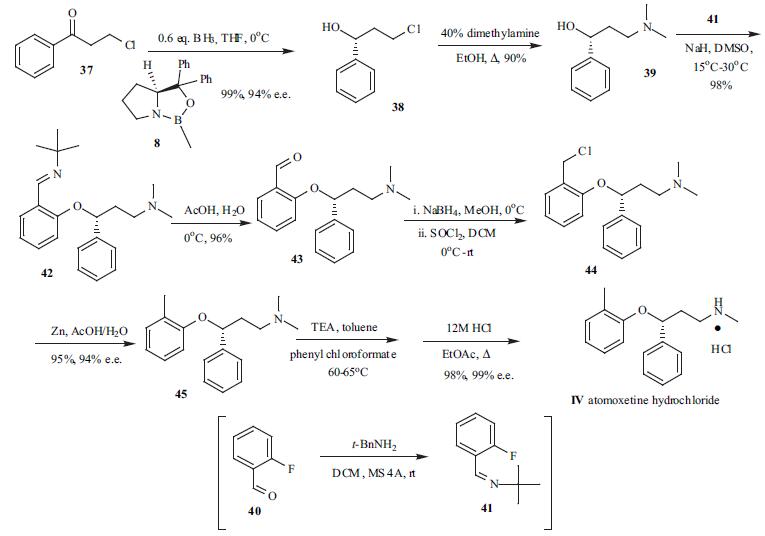
The 3-aryloxy substituent was introduced utilizing a chiral alcohol by either the Mitsunobu reaction or by nucleophilic aromatic displacement. Because of the expense and difficulty of the Mitsunobu reaction on large scale, the commercial process adopts the nucleophilic aromatic substitution method. 3- Chloropropiophenone (37) was asymmetrically reduced with borane and catalytic amount of (S)-oxazaborolidine (8) in THF at 0??C to give chiral alcohol 38 in 99% yield and 94% e.e. The chiral alcohol was further purified by recrystallization to greater than 99% e.e.. Subsequent treatment of chloride 38 with excess dimethylamine (40% in water) in ethanol gave dimethylamine alcohol 39 in 90% yield. Alcohol 39 was then subjected to nucleophilic aromatic displacement in the presence of NaH in DMSO with 1- fluoro-2-(t-butylimino)benzene (41), which was prepared in high yield from 2-fluorobenzaldehyde (40). The displacement product 42 was obtained in 98% yield, and the imine 42 was subsequently hydrolyzed with acetic acid in water at low temperature to give the corresponding aldehyde 43 in 96% yield. Sodium borohydride was employed to reduce aldehyde 43 to alcohol in cold methanol and the intermediate alcohol was converted to chloride 44 with thionyl chloride. Chloride 44 was then reduced with zinc metal under acidic conditions to give methyl adduct 45 in 95% yield and 94% e.e. Finally, phenyl chloroformate and triethylamine was used to transform dimethylamine 45 to monomethyl amine, which was subsequently treated with HCl in EtOAc under reflux to give atomoxetin hydrochloride (IV) in 98% yield and 99% e.e. from 45.
storage
Store at RT
Properties of Atomoxetine hydrochloride
| Melting point: | 167-169°C |
| alpha | D25 -38.01°; 36525 -177.26° (c = 1 in methanol); D23 -41.37° (c = 1.02 in methanol); D25 -40.3° (c = 0.94 in ethanol) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | soluble in Methanol |
| form | solid |
| pka | 10.13(at 25℃) |
| color | White to Almost white |
| Water Solubility | Soluble to 50 mM in water with gentle warming |
| Merck | 14,863 |
| InChI | InChI=1/C17H21NO.ClH/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15;/h3-11,17-18H,12-13H2,1-2H3;1H/t17-;/s3 |
| CAS DataBase Reference | 82248-59-7(CAS DataBase Reference) |
Safety information for Atomoxetine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Atomoxetine hydrochloride
| InChIKey | LUCXVPAZUDVVBT-UNTBIKODSA-N |
| SMILES | O([C@H](CCNC)C1C=CC=CC=1)C1=CC=CC=C1C.Cl |&1:1,r| |
Atomoxetine hydrochloride manufacturer
Unichem Laboratories Ltd
Vasoya Industries Pvt Ltd
Sharon Bio Medicine Ltd
Zydus Lifesciences Ltd.
Ralington Pharma
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 82248-59-7 98%View Details
82248-59-7 98%View Details
82248-59-7 -
 Atomoxetine Hydrochloride (R)-N-Methyl-(2-methylphenoxy)benzenepropanamine Hydrochloride 82248-59-7 96%View Details
Atomoxetine Hydrochloride (R)-N-Methyl-(2-methylphenoxy)benzenepropanamine Hydrochloride 82248-59-7 96%View Details
82248-59-7 -
 Atomoxetine Hydrochloride CAS 82248-59-7View Details
Atomoxetine Hydrochloride CAS 82248-59-7View Details
82248-59-7 -
 82248-59-7 (R)-tomoxetine hydrochloride 98%View Details
82248-59-7 (R)-tomoxetine hydrochloride 98%View Details
82248-59-7 -
 82248-59-7 98%View Details
82248-59-7 98%View Details
82248-59-7 -
 82248-59-7 (R)-tomoxetine hydrochloride 98%View Details
82248-59-7 (R)-tomoxetine hydrochloride 98%View Details
82248-59-7 -
 Atomoxetine hydrochloride 98% (HPLC) CAS 82248-59-7View Details
Atomoxetine hydrochloride 98% (HPLC) CAS 82248-59-7View Details
82248-59-7 -
 Atomoxetine hydrochloride CAS 82248-59-7View Details
Atomoxetine hydrochloride CAS 82248-59-7View Details
82248-59-7