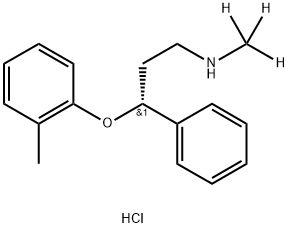
Atomoxetine EP Impurity C HCl
- CAS NO.:1643684-06-3
- Empirical Formula: C17H21NO
- Molecular Weight: 255.35
- MDL number: MFCD06804608

What is Atomoxetine EP Impurity C HCl?
The Uses of Atomoxetine EP Impurity C HCl
p-Tolyloxy Atomoxetine Hydrochloride is an impurity of Atomoxetine Hydrochloride(A791400) which is a norepinephrine reuptake inhibitor.
Pharmacokinetics
Atomoxetine is well absorbed from the GItract and cleared primarily by metabolism, with the majority of the dose being excreted into the urine. Atomoxetine is metabolized primarily by CYP2D6 to its major active metabolite, 4-hydroxyatomoxetine, which is eliminated as its glucuronide. Peak plasma concentrations of atomoxetine occur 1 to 2 hours after oral administration. Significant differences are seen in the elimination half-life between normal metabolizers, extensive metabolizers, and poor metabolizers. Atomoxetine exhibited an elimination half-life of 3 to 6 hours for normal and extensive metabolizers and 17 to 21 hours for poor metabolizers. CYP2C19 is the other enzyme primarily responsible for the formation of its minor metabolite N-desmethylatomoxetine.
Clinical Use
Atomoxetine is used as a safe and well-tolerated “nonstimulant” treatment of ADHD in both adults and children and of depression. Among children and adolescents aged 8 to 18 years, atomoxetine was superior to placebo in reducing symptoms of ADHD and in improving social and family functioning symptoms. Oral atomoxetine is promoted as an alternative to conventional ADHD therapy with methylphenidate, dextroamphetamine, and pemoline. It also can be a replacement for bupropion or for TCAs. Onset of action is approximately 7 days.
Side Effects
At therapeutic doses, no serious drug-related adverse effects have been encountered. Adverse effects have included modest increases in diastolic blood pressure and heart rate, anorexia, weight loss, somnolence, dizziness, GI effects (nausea), dry mouth, and skin rash.
Enzyme inhibitor
This ADHD drug (FWfree-base = 255.36 g/mol; FWhydrochloride = 291.81 g/mol; CAS 83015-26-3), also known as LY139603, Strattera?, and systematically as (3R) -N-methyl-3- (2-methylphenoxy) -3-phenylpropan-1-amine; (R) -N- methyl-3-phenyl-3- (o-tolyloxy) -propan-1-amine, potently inhibits the presynaptic norepinephrine transporter, Ki = 4.5 nM and is used to treat depression and attention-deficit/hyperactivity disorder. In 2002, Strattera became the first FDA-approved nonstimulant for the treatment of ADHD. The mechanism of action is related to its selective inhibition of presynaptic norepinephrine reuptake in the prefrontal cortex. Atomoxetine demonstrates high affinity and selectivity for norepinephrine transporters, but little or no affinity for neurotransmitter receptors. Atomoxetine demonstrates preferential binding to areas of known high distribution of noradrenergic neurons, such as the fronto-cortical subsystem. Atomoxetine undergoes extensive biotransformation, which is affected by poor metabolism by cytochrome P450 (CYP2D6) in a small percentage of the population. (Note: Atomoxetine was originally named tomoxetine, but was changed to avoid any potential confusion with tamoxifen.) Key Pharmacokinetic Parameters: See Appendix II in Goodman & Gilman’s THE PHARMACOLOGICAL BASIS OF THERAPEUTICS, 12th Edition (Brunton, Chabner & Knollmann, eds.) McGraw-Hill Medical, New York.
Properties of Atomoxetine EP Impurity C HCl
| pka | pKa 10.1 (Uncertain) |
Safety information for Atomoxetine EP Impurity C HCl
Computed Descriptors for Atomoxetine EP Impurity C HCl
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1