Irinotecan hydrochloride
Synonym(s):(S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinolin-9-yl ester;[1,4′-Bipiperidine]-1′-carboxylic acid;CPT-11
- CAS NO.:100286-90-6
- Empirical Formula: C33H39ClN4O6
- Molecular Weight: 623.15
- MDL number: MFCD01862255
- EINECS: 600-054-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
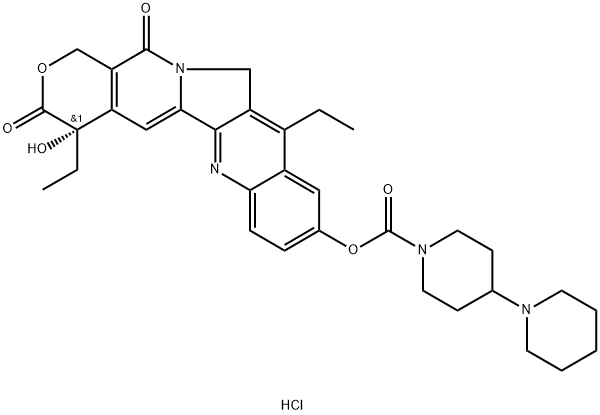
What is Irinotecan hydrochloride?
Description
lrinotecan hydrochloride, a semi-synthetic, water soluble derivative of the potent anticancer agent camptothecin, was launched in Japan for the treatment of lung, ovarian, and cervical cancers. lrinotecan exerts its antitumor activity via inhibition of topoisomerase I, a cellular enzyme that is involved in maintaining the topographic structure of DNA during the process of translation, transcription, and mitosis. lrinotecan undergoes de-esterification in vivo to yield an active metabolite, SN-38, which is 1000-fold more potent than the parent. Although being much less toxic than camptothecin, a significant number of patients in clinical trials exhibited side effects of leukopenia, diarrhea, nauseahromiting, and alopecia. Combination therapy of irinotecan with another widely used anticancer agent, cisplatin, has been reported to be superior to either agent alone. lrinotecan is in clinical trials for gastrointestinal, breast, skin, colorectal, pancreatic cancers, mesothelioma and non-Hodgkin's lymphoma.
Chemical properties
Yellow Crystalline Powder
Originator
Yakult Honsha (Japan)
The Uses of Irinotecan hydrochloride
Irinotecan hydrochloride has been used:
- in combination with 5-fluorouracil for screening growth inhibitory functionality in MDA-MB-231 breast cancer cells.
- in chemosensitivity screening of high-grade appendiceal (HGA) and low-grade appendiceal (LGA) organoids.
- as a chemotherapeutic agent in the cytotoxicity studies in combination with heat shock proteins inhibitors (HPSC1) in HT29 colon cancer cells.
The Uses of Irinotecan hydrochloride
A DNA topoisomerase inhibitor
The Uses of Irinotecan hydrochloride
antineoplactic;'inhibitor of topoisomerase I
Definition
ChEBI: A hydrochloride obtained by combining irinotecan with one molar equivalent of hydrochloric acid. Used (in the form of its trihydrate) in combination with fluorouracil and leucovorin, for the treatment of patients with metastatic adenocarcinoma of the pancr as after disease progression following gemcitabine-based therapy. It is converted via hydrolysis of the carbamate linkage to its active metabolite, SN-38, which is ~1000 times more active.
Manufacturing Process
7-Ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin was
synthesized by 2 methods.
Method 1.
7-Ethyl-10-hydroxycamptothecin (500 mg, 1.27 mmol) was suspended in dry
dioxane (400 ml) and dissolved therein by adding triethylamine (2 ml) to the
suspension under warming. This solution was stirred at room temperature
while introducing thereinto phosgene prepared toties quoties by decomposing
phosgene dimer (trichloromethoxychloroformate, 400 ml) in the presence of
an active carbon catalyst. After 0.5 hours, consumption of the starting
materials was confirmed and insoluble 10-chlorocarbonyloxy-7-
ethylcamptothecin was removed by filtration.
10-Chlorocarbonyloxy-7-ethylcamptothecin (300 mg, 0.66 mmol) is
suspended in dry dioxane (50 ml). To this suspension is added 4-
piperidinopiperidine (330 mg, 1.96 mmol) as the amine, the reaction followed
by the after-treatment was carried out whereby the 7-ethyl-10-[4-(1-
piperidino)-1-piperidino]carbonyloxycamptothecin title compound (154 mg,
39.8%) was obtained.
Method 2.
7-Ethyl-10-hydroxycamptothecin (790 mg, 2.01 mmol) and 1-chlorocarbonyl-
4-piperidinopiperidine (910 mg, 3.95 mmol) were dissolved in anhydrous
pyridine (50 ml), and the mixture was stirred for 1 hour at 20°C. The reaction
mixture was evaporated to dryness in vacuo, the residue was dissolved in
CHCl3 (200 ml). The solution was washed successively with a 7% aqueous
solution of NaHCO3 (200 ml), a saturated aqueous solution NaCl, and the
CHCl3 layer was filtered, and evaporated in vacuo. The residual material was
decolorized by passing it through a short silica gel column. 7-Ethyl-10-[4-(1-
piperidino)-1-piperidino]carbonyloxycamptothecin was obtained as a pale
yellow mass, which was recrystallized from ethanol (ca. 60 ml) to give
colorless needles (750 mg, 63.5% in yield).
To an ice-cooled suspension in distilled water (15 ml) of 7-ethyl-10-[1-(4-
piperidino)piperidino]carbonyloxycamptothecin (1.00 g, 1.7 mmol) was added
0.1 N HCl (15.3 ml, 1.53 mmol), and the suspension was stirred vigorously
for 5 minutes under cooling in an ice bath and filtered off. 7-Ethyl-10-[4-(1-
piperidino)-1-piperidino]carbonyloxycamptothecin hydrochloride was obtained
in yield 96%.
brand name
Camptosar (Pharmacia &Upjohn) ;Topotecin.
Therapeutic Function
Antineoplastic
General Description
Irinotecan is available in 100-mg or 5-mL vials for IV administrationand is used in combination with 5-FU and leucovorinas first-line treatment of metastatic colon cancer.The agent may also be used as a single agent in colorectalcancer as a second-line therapy when 5-FU therapy hasfailed. Additional uses include small cell lung cancer,NSCLC, cervical cancer, esophageal cancer, and gastric cancer Irinotecan is 30% to 60% plasma protein bound, whereasthe active metabolite SN-38 is 95% protein bound. Bindingof SN-38 as the lactone stabilizes the material to ring opening.The elimination of the agent occurs primarily in the bilewith a minor amount of renal elimination. The excretion ofactive metabolites or inactive metabolites such as the glucuronideSN-38G, which may be converted back to SN-38 inthe bile, has been associated with severe diarrhea. Irinotecanand SN-38 have half-lives of 8 and 14 hours, respectively.Irinotecan has two dose-limiting toxicities, myelosuppressionand diarrhea. The diarrhea occurs in two forms, earlyand late. The early form occurs within the first 24 hours afteradministration. It has been associated with inhibition ofacetylcholinesterase, which results in increased gut motility.This early phase is also associated with flushing, abdominalpain, and excessive sweating. Atropine can be used to relievethese symptoms but it is not recommended for prophylacticuse unless there has been a prior episode. The late-phasediarrhea occurs after 24 hours and has been associated withthe presence of active material, particularly SN-38 in the gut,and may last 3 to 10 days. The prolonged nature may lead todehydration and electrolyte imbalances. Loperamide therapyis recommended at the first appearance of a loose stool. If thediarrhea persists, additional agents may be used includingantibiotics that decrease β-glucosidase–producing bacteria inthe gut and prevent the overgrowth of pathogenic bacteria.111Other toxicities include emesis and alopecia.
Biological Activity
Inhibitor of DNA topoisomerase I that displays antitumor activity against a range of tumor types.
Biochem/physiol Actions
The anticancer agent, irinotecan, is a prodrug that is converted by tissue carboxylesterase to 7-ethyl-10-hydroxycamptothecin (SN-38), a potent inhibitor of DNA topoisomerase I. Its action is terminated by glucuronidation by UDP glucuronosyl transferase 1A1 (UGT1A1). It proves useful in radiation treatment of tumors by sensitizing tissue to radiation damage.
Clinical Use
In combination with fluorouracil, this prodrug camptothecin analogue is considered to be first-line therapy in the treatment of metastatic colorectal cancer. It also has shown efficacy in small cell and nonsmall cell lung cancers when used in combination with cisplatin.
Side Effects
Delayed diarrhea induced by irinotecan is dose-limiting and potentially fatal, and vigorous loperamide therapy should be instituted at the first sign of symptoms. Acute diarrhea is attributed to the drug's ability to inhibit acetylcholinesterase and can be addressed through anticholinergic pretreatment. Pretreatment also helps patients to avoid “cholinergic syndrome,” a collection of annoying side effects that include flushing, sweating, blurred vision, lacrimation, and less commonly, bradycardia. Camptothecins also are myelosuppressive, and neutropenia can be severe, particularly in patients with elevated bilirubin levels. Extensive biotransformation also demands cautious use of irinotecan in patients with hepatic dysfunction.
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: concentration reduced by St John’s
wort - avoid.
Antifungals: increased toxicity with itraconazole -
avoid; concentration reduced by ketoconazole, but
active metabolite of irinotecan increased - avoid.
Antipsychotics: avoid concomitant use with
clozapine (increased risk of agranulocytosis).
Antivirals: metabolism possibly inhibited by
atazanavir (increased risk of toxicity).
Cytotoxics: concentration of active metabolite of
irinotecan increased by lapatinib, consider reducing
dose of irinotecan; avoid with panitumumab;
concentration possibly increased by sorafenib.
Live vaccines: risk of generalised infections - avoid.
Metabolism
The drug is slowly bioactivated in the liver through hydrolysis of the C10-carbamate ester. The catalyzing enzyme is a saturable carboxylesterase known as irinotecan-converting enzyme. Levels of active metabolite, known as SN-38, are 50- to 100-fold lower than the parent drug, but preferential protein binding of the lactone (95%) permits significant plasma levels of the optimally active SN-38 compared to the hydroxy acid metabolite. SN-38 has a terminal half-life of 11.5 hours (compared to 5.0–9.6 hours for the prodrug parent) and is glucuronidated at the C10 phenol before elimination. CYP3A4 also cleaves the terminal piperidine ring through oxidation at the α-carbons, followed by hydrolysis of the resultant amides, producing inactive metabolites. Excretion of the parent drug and metabolites is renal (14–37%) and, to a lesser extent, biliary.
Storage
Store at RT
Properties of Irinotecan hydrochloride
| Melting point: | 250-256°C (dec.) |
| Boiling point: | 257 °C |
| refractive index | 67.7 ° (C=1, H2O) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO or DMF at approximately 20mg/ml. Sparingly soluble in aqueous buffers.
/n |
| form | Yellow powder |
| color | White to yellow |
| Water Solubility | Soluble in DMSO at 100mg/ml. Soluble in water at 25mg/ml with warming |
| Merck | 5091 |
| CAS DataBase Reference | 100286-90-6(CAS DataBase Reference) |
Safety information for Irinotecan hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. |
Computed Descriptors for Irinotecan hydrochloride
Irinotecan hydrochloride manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![sodium:(2S)-2-[12-ethyl-8-(hydroxymethyl)-9-oxo-2-(4-piperidin-1-ylpiperidine-1-carbonyl)oxy-11H-indolizino[1,2-b]quinolin-7-yl]-2-hydroxybutanoate](https://img.chemicalbook.in/CAS/20180527/GIF/1329502-92-2.gif)


You may like
-
 100286-90-6 99%View Details
100286-90-6 99%View Details
100286-90-6 -
 Irinotecan hydrochloride 99%View Details
Irinotecan hydrochloride 99%View Details
100286-90-6 -
 Irinotecan hydrochloride 100286-90-6 98%View Details
Irinotecan hydrochloride 100286-90-6 98%View Details
100286-90-6 -
 Irinotecan hydrochloride 98%View Details
Irinotecan hydrochloride 98%View Details
100286-90-6 -
 100286-90-6 99%View Details
100286-90-6 99%View Details
100286-90-6 -
 Irinotecan hydrochloride, ≥97% CAS 100286-90-6View Details
Irinotecan hydrochloride, ≥97% CAS 100286-90-6View Details
100286-90-6 -
 Irinotecan hydrochloride CAS 100286-90-6View Details
Irinotecan hydrochloride CAS 100286-90-6View Details
100286-90-6 -
 Irinotecan HCl 99.50%View Details
Irinotecan HCl 99.50%View Details
100286-90-6
