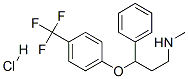
Fluoxetine hydrochloride
Synonym(s):(±)-N-Methyl-γ-[4-(trifluoromethyl)phenoxy]benzenepropanamine hydrochloride;Fluoxetine HCl;Fluoxetine hydrochloride;FLX;Prozac
- CAS NO.:56296-78-7
- Empirical Formula: C17H19ClF3NO
- Molecular Weight: 345.79
- MDL number: MFCD00214288
- EINECS: 260-101-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
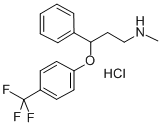
What is Fluoxetine hydrochloride?
Description
Fluoxetine hydrochloride is a selective serotonin reuptake inhibitor (SSRI), it is adapted to the treatment of various depressive disorders, including mild or major depression, bipolar disorder depression, psychogenic depression and depressive neurosis. The mechanism is inhibiting neuronal uptake of serotonin from the synaptic cleft, increasing this neurotransmitter in the gap for practical use , thereby improving the emotional state,and treating depressive disorders. It also appears effective in the treatment of obesity.
Chemical properties
White to Off-White Powder
Originator
Lilly (USA)
The Uses of Fluoxetine hydrochloride
Fluoxetine hydrochloride is a selective serotonin reuptake inhibitor. It is used to treat major depressive disorder, bulimia nervosa (an eating disorder) obsessive-compulsive disorder, panic disorder and premenstrual dysphoric disorder (PMDD).
What are the applications of Application
Fluoxetine hydrochloride is a selective serotonin reuptake inhibitor that reverses depressive-like behavior induced by unpredictable chronic mild stress.
Definition
ChEBI: Fluoxetine hydrochloride (1:1) is a hydrochloride and a N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine. It inhibits the reuptake of serotonin and is used chiefly as an antidepressant.
What are the applications of Application
Fluoxetine hydrochloride has been used to study its effect on the binding ability of the radiopharmaceutical 123I-labeled 2-((2-((dimethylamino)methyl)phenyl)thio)-5-iodophenylamine ([123I]ADAM) to SERT (serotonin transporters) in mice. It has also been used for the chronic treatment of light deprived animals.
Preparation
A method for the synthesis of fluoxetine hydrochloride was developed by the Mannich reaction of acetophenone with methylamine hydrochloride, paraformaldehyde to form 3-methylamino-1-phenylacetic acid hydrochloride, followed by the preparation of 3-methylamino-I-phenylpropanol in methanol with potassium borohydride reductant, followed by etherification and salt formation to synthesize the antidepressant fluoxetine monohydrochloride.
Fluoxetine is the active ingredient in the antidepressant Prozac. It works as a selective serotonin reuptake inhibitor to treat conditions including depression and obsessive-compulsive disorder. To assemble this molecule, a three-step synthesis was utilized. Intermediates included 1- propanone, 3-(methylamino)-1-phenyl-(synthesized through an SN2 reaction between 3-chloropropiophenone and methylamine) and α-[2- (methylamino) ethyl] benzyl alcohol (synthesized through reduction of the first intermediate using NaBH4). The second intermediate was subjected to 4-chlorobenzotrifluoride and sodium hydride to produce the desired molecule, fluoxetine.
brand name
Prozac (Lilly); Sarafem (Lilly); Sarafem (Warner Chilcott).
Therapeutic Function
Antidepressant
General Description
Fluoxetine hydrochloride is an antidepressant drug and a selective serotonin reuptake inhibitor, which is widely used for the treatment of major depressive disorders, obsessive-compulsive disorder and panic fits.
Biological Activity
Selective serotonin reuptake inhibitor. Binds to the human 5-HT transporter with a K i of 0.9 nmol/l and is between 150- and 900- fold selective over 5-HT 1A , 5-HT 2A , H 1 , α 1 , α 2 -adrenergic, and muscarinic receptors. Antidepressant. Also available as part of the Serotonin Uptake Inhibitor Tocriset™ .
Biochem/physiol Actions
Selective serotonin reuptake inhibitor; antidepressant.
Side Effects
Common adverse reactions are dry mouth, loss of appetite, nausea, insomnia, fatigue, a few cases of anxiety and headaches are seen . Because fluoxetine hydrochloride has longer half-life, patients whose liver and kidney function are poor or elderly patients, should appropriately reduce the dose. Children should be applied in accordance with the doctor's advice. Patients who has the history of epilepsy, pregnancy or lactation women should use with caution . If rash or fever occurs, the drug should be discontinued immediately and symptomatic treatment should be given. It is not appropriate to be used with monoamine oxidase inhibitor (MAOI) ; if necessary, the drug should be discontinued after five weeks, before switching to a monoamine oxidase inhibitor (MAOI).
in vitro
in xenopusoocytes expressing either cloned 5ht2c receptors or 5ht receptors, micromolar concentrations of fluoxetine (prozac) inhibited the membrane currents elicited by serotonin (5-hydroxytryptamine; 5ht). for responses elicited by 1 μm 5-ht, the ic50 of fluoxetine was about 20 μm. fluoxetine also inhibited the binding of [3h]5ht to 5ht2c receptors expressed in hela cells and the binding of [3h]5ht to 5ht receptors in rat cortex membranes, with ki of ≈65–97 nm and ≈ 56 μm, respectively[2]. administration of fluoxetine blocked the downregulation of cell proliferation of hippocampal cells resulting from inescapable shock (is), which resulted in a state of behavioral despair[3]. fluoxetine increased the number of newborn cells in the dentate gyrus of the hippocampus of adult rat. fluoxetine also increased the number of proliferating cells in the prelimbic cortex[4]. fluoxetine accelerated the maturation of immature neurons. fluoxetine enhanced neurogenesis-dependent long-term potentiation (ltp) in the dentate gyrus [5]. fluoxetine, but not other selective serotonin uptake inhibitors such as citalopram, fluvoxamine, paroxetine and sertraline, increased norepinephrine and dopamine extracellular levels in prefrontal cortex. fluoxetine produced robust and sustained increases in extracellular concentrations of norepinephrine and dopamine after acute systemic administration [6].
in vivo
fluoxetine reversed the deficit in escape latency observed in animals exposed to inescapable shock in adult male sprague–dawley rats [3].the combination of olanzapine and fluoxetine produced robust, sustained increases of extracellular levels of dopamine ([da](ex)) and norepinephrine ([ne](ex)) up to 361 ± 28% and 272 ± 16% of the baseline, respectively[7]. fluoxetine (5 and 10 mg/kg) reduced cocaine infusions (0.2 mg/kg), and cocaine infusions returned to baseline levels within 48 hr after fluoxetine treatments were terminated [8]. in sham-operated or adrenalectomized/castrated (adx/cx) male rats, administration of fluoxetine dose-dependently (2.9-58 mumol/kg i.p.) increased the brain content of the neurosteroid 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone, 3 alpha, 5 alpha-th prog)[9].
Storage
Room temperature
References
1) Tatsumi?et al.?(1997),?Pharmacological profile of antidepressants and related compounds at human monoamine transporters; Eur. J. Pharmacol.,?340?249 2) Benfield?et al.?(1986),?Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness; Drugs,?32?481 3) Benvenuto?et al.?(2013),?Pharmacotherapy of autism spectrum disorders; Brain Dev.,?35?119 4) Liu?et al.?(2018),?Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NFkB signaling pathway; J. Neuroinflammation,?15?347 5) Chang?et al.?(2010),?Increased cellular turnover in response to fluoxetine in neuronal precursors derived from human embryonic stem cells; Prog. Int. J. Dev. Biol.,?54?707
Properties of Fluoxetine hydrochloride
| Melting point: | 158-159°C |
| alpha | -0.05~+0.05° |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: soluble (sparingly) |
| form | solid |
| color | white |
| Water Solubility | Soluble in dimethylsulfoxide and water. |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 56296-78-7(CAS DataBase Reference) |
| EPA Substance Registry System | Fluoxetine hydrochloride (56296-78-7) |
Safety information for Fluoxetine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Fluoxetine hydrochloride
| InChIKey | GIYXAJPCNFJEHY-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 56296-78-7 FLUOXETINE HCL IP/USP 99%View Details
56296-78-7 FLUOXETINE HCL IP/USP 99%View Details
56296-78-7 -
 Fluoxetine hydrochloride 98%View Details
Fluoxetine hydrochloride 98%View Details
59333-67-4 / 56296-78-7 -
 59333-67-4 / 56296-78-7 Fluoxetine hydrochloride 99%View Details
59333-67-4 / 56296-78-7 Fluoxetine hydrochloride 99%View Details
59333-67-4 / 56296-78-7 -
 59333-67-4 / 56296-78-7 Fluoxetine hydrochloride 98%View Details
59333-67-4 / 56296-78-7 Fluoxetine hydrochloride 98%View Details
59333-67-4 / 56296-78-7 -
 Luoxetine HCl Reference Standard 56296-78-7 98%View Details
Luoxetine HCl Reference Standard 56296-78-7 98%View Details
56296-78-7 -
 Fluoxetine Hydrochloride CAS 56296-78-7View Details
Fluoxetine Hydrochloride CAS 56296-78-7View Details
56296-78-7 -
 Fluoxetine CAS 56296-78-7View Details
Fluoxetine CAS 56296-78-7View Details
56296-78-7 -
 Fluoxetine hydrochloride CAS 56296-78-7View Details
Fluoxetine hydrochloride CAS 56296-78-7View Details
56296-78-7
