Apixaban
- CAS NO.:503612-47-3
- Empirical Formula: C25H25N5O4
- Molecular Weight: 459.5
- MDL number: MFCD11977295
- EINECS: 639-684-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 14:33:46
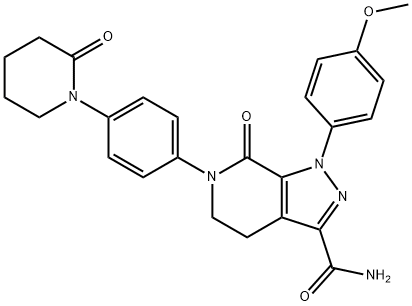
What is Apixaban?
Absorption
Apixaban is approximately 50% bioavailable though other studies report 43-46% oral bioavailability.
Toxicity
Animal studies have shown an increased risk of maternal bleeding during pregnancy but no increase in fetal malformations or fetal or maternal deaths. It is unknown if this animal data also translates to humans so apixaban should only be used in pregnancy if the benefits outweigh the risks.
It is not know whether apixaban is safe and effective in labor and during birth, though animal studies have shown an increased rate of maternal bleeding. Animal studies in rats show apixaban excreted in milk, though it is not know if this also applies to humans. Nursing mothers should either stop breastfeeding or stop taking apixaban depending on the risk and benefit of each option.
Studies to determine safety and effectiveness in pediatric patients have yet to be performed.
Studies that involved geriatric patients (at least 75 years old) saw no difference in safety or effectiveness compared to younger patients, though geriatric patients at an especially advanced age may be more susceptible to adverse effects. Dosage adjustments for patients with end stage renal disease(ESRD) are based on estimates of pharmacokinetic principles and not clinical study. Patients with ESRD may experience pharmacodynamics similar to those seen in well controlled studies but it may not lead to the same clinical effects. Dosage adjustments are not necessary in mild hepatic impairment. In moderate hepatic impairment patients may already experience abnormalities in coagulation and so no dose recommendations are possible. Apixaban is not recommended for patients with severe hepatic impairment.
Description
Eliquis (apixaban), a direct inhibitor of factor Xa (FXa), was approved by the European Commission on May 18, 2011 for prevention of venous thromboembolic events (VTE) in adult patients who have undergone elective hip or knee replacement surgery. The discovery of apixaban was the culmination of a succession of novel and innovative medicinal chemistry discoveries starting with the identification of nonpeptide leads, rational drug design using computer-aided and X-ray crystallographic information, and the building of drug-like properties through the systematic replacement of basic groups with neutral moieties. Apixaban arose from modifications to razaxaban by constraining a pyrazole amide to form a bicyclic pyrazolo-pyridinone scaffold. Optimization of the P1 group resulted in the identification of the nonbasic methoxy phenyl group, while a P4 piperidinone improved the balance of potency and pharmacokinetics with low Vdss. The synthesis of apixaban begins with the generation of a hydrazone of 4-methoxyaniline which is then used in a 3+2 cycloaddition with a dihydropiperidinone to form a bicyclic pyrazolo-pyridinone scaffold. The distal piperidinone group is installed using an Ullmann coupling reaction followed by aminolysis of an ethyl ester on the pyrazole ring to complete the synthesis of apixaban.
Description
Apixaban1 is an anticoagulant drug that was first described in several mid-2000s patent applications.
BMS and Pfizer (New York) codeveloped apixaban under the brand name Eliquis. The drug was approved for use by the European Medicines Agency in 2011 and by the US Food and Drug Administration in 2012.
Apixaban is used to treat or prevent blood clots, including deep vein thrombosis, which can lead to pulmonary embolism. It can also prevent stroke in patients with nonvalvular atrial fibrillation. It is often given to patients after hip or knee replacement surgery to prevent clotting during recovery. It is administered orally and is safer and more convenient than warfarin, the medication previously used for these purposes.
Description
Apixaban is an orally bioavailable, selective inhibitor of both free and prothrombinase-bound factor Xa (Kis = 0.8 nM). In vivo, apixaban has antithrombotic effects in a rabbit model of venous thrombosis. Apixaban (357.5 mg) also prevents thrombus formation without inducing adverse bleeding events in a porcine model of aortic heterotopic valve replacement. Formulations containing apixaban have been used to prevent blood clot formation in patients with atrial fibrillation.
Originator
Bristol Myers Squibb Company (United States)
The Uses of Apixaban
Apixaban is a highly selective, reversible inhibitor of Factor Xa with Ki of 0.08 nM and 0.17 nM in human and rabbit, respectively.
What are the applications of Application
Apixaban is a highly selective, reversible, and direct inhibitor of Factor X
Background
Apixaban is an oral, direct, and highly selective factor Xa (FXa) inhibitor of both free and bound FXa, as well as prothrombinase, independent of antithrombin III for the prevention and treatment of thromboembolic diseases. It is marketed under the name Eliquis. Apixaban was approved by the FDA on December 28, 2012.
Indications
Apixaban is indicated for reducing the risk of stroke and systemic embolism in patients who have nonvalvular atrial fibrillation, prophylaxis of deep vein thrombosis(DVT) leading to pulmonary embolism(PE) in patients after a hip or knee replacement surgery, and treatment of DVT and PE to reduce the risk of recurrence.
Definition
ChEBI: A pyrazolopyridine that is 7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide substituted at position 1 by a 4-methoxyphenyl group and at position 6 by a 4-(2-oxopiperidin-1-yl)phenyl group. It is used for the prevention and treatment of thromboembolic diseases.
brand name
Eliquis
Pharmacokinetics
The maximum plasma concentration (Cmax) of apixaban occurs 3–4 h after oral administration. The absorption of apixaban appears to occur primarily in the small intestine and decreases progressively throughout the gastrointestinal tract. Compared with oral administration, the bioavailability of 2.5 mg of apixaban solution was approximately 60% and 84% lower when released in the distal small bowel and ascending colon, respectively. For oral doses up to 10 mg, the absolute bioavailability of apixaban is~50%, resulting from the incomplete absorption and first-pass metabolism in the gut and liver[1].
Clinical Use
Apixaban is an oral anticoagulant with highly selective inhibition of factor Xa. It was approved by the European Medicines Agency (EMA) for the treatment of venous thromboembolic events and first marketed in Germany under the brand name Eliquis in June 2011. Apixaban was co-developed by Bristol-Myers Squibb and Pfizer and represents the first approved drug for this indication since warfarin over 50 years ago.
Side Effects
Possible side effects of Apixaban are: bleeding gums, nosebleeds, heavy vaginal bleeding , red, pink, or brown urine; red or black, tarry stools; coughing or spitting up blood or a substance that looks like coffee grounds; swelling or joint pain, headache, rash, chest pain or tightness in the chest, swelling of the face or tongue, trouble breathing, wheezing. Feeling dizzy or fainting.Apixaban prevents your blood from clotting properly, so if you get a cut or injury, it may take longer than usual for the bleeding to stop. This medication may also cause you to bruise or bleed more easily.
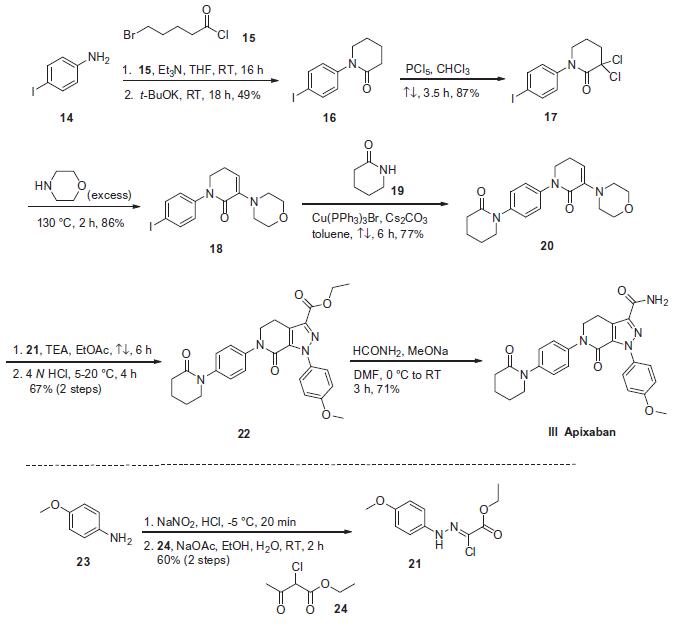
Synthesis
Although several convenient preparations of apixaban (BMS-562247) have been reported, the most likely process-scale route is described in the scheme. The starting material 4-iodoaniline (14) was acylated with 5-bromovaleryl chloride (15) and triethylamine followed by cyclization under basic conditions to give lactam 16 in 49% yield. Intermediate 16 was then reacted with phosphorus pentachloride to provide the a,a-dichlorinated lactam 17 in 87% yield.30 This dichloride was reacted with excess morpholine to affect an alkylation¨Celimination sequence to afford enaminolactam 18 in 86% yield. N-Arylation of this iodide with valerolactam 19 using a copper (I) catalyst resulted in a 77% yield of the desired p-bispiperidone 20. Interestingly, sequential exposure of 20 to a nitrile imine generated from the treatment of ethyl 2-chloro-2-(2,4-methoxyphenyl)-hydrazono) acetate 21 with base resulted in a [3+2] dipolarcycloadditon reaction. Upon acidification with 4 N HCl, pyrazole 22 was furnished in 67% over two steps. Conversion of the ester within 22 to the corresponding amide was achieved via a mixture of formamide and sodium methoxide to give apixaban (III) in 71% yield. It is important to note that intermediate 21 was prepared from commercially available 4-methoxyaniline (23) by sequential diazotization and condensation with ethyl 2-chloroacetoacetate (24).
in vitro
apixabanhas exhibited a high degree of potency, selectivity, and efficacy on factor xa with ki of 0.08 nm and 0.17 nm for human factor xa and rabbit factor xa, respectively [1]. apixaban prolonged the clotting times of normal human plasma with the concentrations (ec2x) of 3.6, 0.37, 7.4 and 0.4 μm, which are required respectively to double the prothrombin time (pt), modified prothrombin time (mpt), activated partial thromboplastin time (aptt) and heptest. besides, apixaban showed the highest potency in human and rabbit plasma, but less potency in rat and dog plasma in both the pt and aptt assays [3].
in vivo
apixaban exihibited the excellent pharmacokinetics with very low clearance (cl: 0.02 l kg-1h-1), and low volume of distribution (vdss: 0.2 l/kg) in the dog. besides, apixaban also showed a moderate half-life with t1/2 of 5.8 hours and good oral bioavailability (f: 58%) [1]. in the arteriovenous-shunt thrombosis (avst), venous thrombosis (vt) and electrically mediated carotid arterial thrombosis (ecat) rabbit models, apixaban produced antithrombotic effects with ec50 of 270 nm, 110 nm and 70 nm in a dose-dependent manner[3]. apixaban significantly inhibited factor xa activity with an ic50 of 0.22 μm in rabbit ex vivo[4]. in chimpanzee, apixaban also showed small volume of distribution (vdss: 0.17 l kg-1), low systemic clearance (cl: 0.018 l kg-1h-1), and good oral bioavailability (f: 59%) [5].
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: increased risk of haemorrhage with IV
diclofenac and ketorolac - avoid.
Antibacterials: avoid with clarithromycin and
telithromycin; concentration possibly reduced by
rifampicin - avoid if treating DVT/PE.
Anticoagulants: increased risk of haemorrhage with
other anticoagulants - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid if treating DVT/PE.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone - avoid if treating DVT/
PE with carbamazepine.
Antifungals: concentration increased by ketoconazole
- avoid; avoid with itraconazole, posaconazole and
voriconazole.
Antivirals: avoid with atazanavir, boceprevir,
darunavir, fosamprenavir, indinavir, lopinavir,
ritonavir, saquinavir, telaprevir and tipranavir.
Cobicistat: avoid concomitant use.
Pharmacokinetics
Apixaban's mode of action is factor Xa inhibition. Factor Xa is activated by the hydrolysis of factor X, an enzyme that is a component of the coagulation (clotting) cascade. Factor Xa, in turn, catalyzes the conversion of prothrombin to thrombin, the enzyme that promotes the formation of fibrin clots.
Metabolism
50% of the orally administered dose is excreted as the unchanged parent compound, however 25% of the dose is excreted as O-demethyl apixaban sulfate. All apixaban metabolites account for approximately 32% of the excreted dose though the structure of all metabolites are not well defined. Apixaban is mainly metabolized by cytochrome p450(CYP)3A4 and to a lesser extent by CYP1A2, CYP2C8, CYP2C9, CYP2C19, and CYP2J2.
Metabolism
Apixaban is metabolised in the liver mainly via the P450
cytochromes CYP3A4 and CYP3A5.
Apixaban has multiple routes of elimination. Of the
administered apixaban dose in humans, approximately
25% was recovered as metabolites, with the majority
recovered in faeces. Renal excretion of apixaban accounts
for approximately 27% of total clearance. There are also
additional contributions from biliary and direct intestinal
excretion.
References
https://en.wikipedia.org/wiki/Apixaban
https://www.drugbank.ca/drugs/DB06605
[1] Wonkyung Byon. “Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review.” Clinical Pharmacokinetics 58 10 (2019): 1265–1279.
Properties of Apixaban
| Melting point: | 235-238°C |
| Boiling point: | 770.5±60.0 °C(Predicted) |
| Density | 1.42 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| form | Solid |
| appearance | white to pale yellow crystals or powder |
| pka | 15.01±0.20(Predicted) |
| color | White to Off-White |
Safety information for Apixaban
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Apixaban
| InChIKey | QNZCBYKSOIHPEH-UHFFFAOYSA-N |
| SMILES | C1(=O)N(C2=CC=C(N3CCCCC3=O)C=C2)CCC2C(C(N)=O)=NN(C3=CC=C(OC)C=C3)C1=2 |
Apixaban manufacturer
SRINI PHARMACEUTICALS PVT LTD
DR ACHARYA LABORATORIES PVT LTD
Kavya Pharma
Eastern Chemicals Mumbai Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![Ethyl (2Z)-chloro[(4-methoxyphenyl)hydrazono]ethanoate](https://img.chemicalbook.in/CAS2/GIF/473927-63-8.gif)

![1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester](https://img.chemicalbook.in/CAS2/GIF/503614-91-3.gif)
You may like
-
 Apixaban 98%View Details
Apixaban 98%View Details -
 Apixaban 98%View Details
Apixaban 98%View Details -
 Apixaban 99%View Details
Apixaban 99%View Details -
 Apixaban 98%View Details
Apixaban 98%View Details -
 Apixaban 98%View Details
Apixaban 98%View Details -
 Apixaban 98%View Details
Apixaban 98%View Details -
 Apixaban 98%View Details
Apixaban 98%View Details -
 Apixaban 98%View Details
Apixaban 98%View Details
