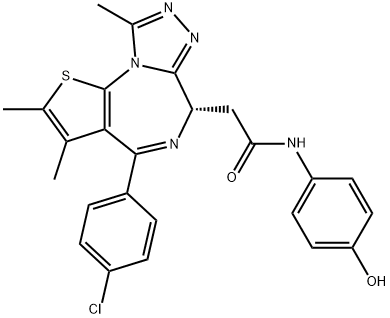
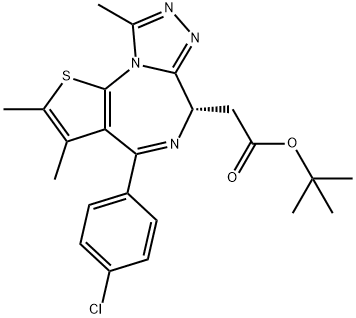
(+)-JQ-1
Synonym(s):(S)-(+)-tert-Butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate;6H-Thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine-6-acetic acid, 4-(4-chlorophenyl)-2,3,9-trimethyl-, 1,1-dimethylethyl ester, (6S)-
- CAS NO.:1268524-70-4
- Empirical Formula: C23H25ClN4O2S
- Molecular Weight: 456.99
- MDL number: MFCD22683748
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-11 10:02:43
What is (+)-JQ-1?
Description
JQ1 (+) (1268524-70-4) is a potent BET bromodomain inhibitor and is the active isomer.?? IC50 = 17.7, 32.6, 76.9 and 12942 nM respectively for BRD2 (N-terminal (N)), BRD4 (C-terminal (C)), BRD4 (N) and CREBBP respectively (data for + isomer).1 Competitive binding by JQ1 displaces the BRD4 fusion oncoprotein from chromatin, prompting squamous differentiation and specific antiproliferative effects in BRD4-dependent cell lines and patient-derived xenograft models.1?Induces squamous differentiation in NMC cell lines and inhibits tumor growth in NMC xenografts.2?Displays reversible contraceptive effects in male mice.3?Blocks inflammation and bone loss in periodontitis.4?Reverses CAR T cell extinction.5
The Uses of (+)-JQ-1
BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation.
The Uses of (+)-JQ-1
(+)-JQ1 has been used in flow cytometry assay, cell viability assay and quantitative PCR assay in order to investigate on the reversal of HIV-1 latency.
Definition
ChEBI: JQ1 is a member of the class of thienotriazolodiazepines that is the tert-butyl ester of [(6S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl]acetic acid. An inhibitor of bromodomain-containing protein 4 that exhibits anti-cancer and cardioprotective properties. It has a role as a bromodomain-containing protein 4 inhibitor, a cardioprotective agent, an antineoplastic agent, an anti-inflammatory agent, an angiogenesis inhibitor, an apoptosis inducer and a ferroptosis inducer. It is a thienotriazolodiazepine, an organochlorine compound, a carboxylic ester and a tert-butyl ester.
General Description
JQ1 is a member of the triazolo-diazepine compound family, which functions as a pan-BET (bromodomain and extra-terminal motif) family inhibitor. JQ1 is known to suppress cell proliferation and therefore, can be used as a therapeutic drug for a number of cancers including multiple myeloma and acute myeloid leukemia.
Biochem/physiol Actions
(+)-JQ1 is a high affinity, potent and selective inhibitor of BET bromodomain proteins, including BRD2, BRD3, BRD4 and BRDT. (+)-JQ1 (also known as SGCBD01), the active enantiomer of (+/-)-JQ1, inhibits Brd4 (bromodomain-containing 4), which forms complexes with chromatin via two tandem bromodomains (BD1 and BD2) that bind to acetylated lysine residues in histones and Brd4 association with acetylated chromatin is believed to regulate the recruitment of elongation factor b and additional transcription factors to specific promoter regions. The nuclear protein in testis (NUT) gene is known to form fusions with Brd4 that create a potent oncogene, leading to rare, but highly lethal tumors referred to as NUT midline carcinomas (NMC). (+)-JQ1 inhibits recruitment and binding of Brd4 to TNFa and E-selectin promoter elements, and accelerates recovery time in FRAP (fluorescence recovery after photobleaching) assays using GFP-Brd4. Thus (+)-JQ1 is a useful tool to study the role of Brd4 in transcriptional initiation.For characterization details of (+)-JQ1, please visit the JQ-1 probe summary on the Structural Genomics Consortium (SGC) website.(-)-JQ1 is the negative control for the active enantiomer, (+)-JQ1. (-)-JQ1 is available from Sigma. To learn more about and purchase (-)-JQ1, click here.To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
storage
-20°C
References
References/Citations:
Properties of (+)-JQ-1
| Melting point: | >205°C (dec.) |
| Boiling point: | 610.4±65.0 °C(Predicted) |
| Density | 1.33 |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| pka | 2.05±0.60(Predicted) |
| form | powder |
| color | white to beige |
| Stability: | Stable for 1 year as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
Safety information for (+)-JQ-1
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (+)-JQ-1
| InChIKey | DNVXATUJJDPFDM-KRWDZBQOSA-N |
New Products
4-Piperidinemethanol Ethyl 2,4-Dihydroxy-6-methylnicotinate Ethyl isonicotinate 3-pyridine methanol N-Methyl 4-chloro-pyridine-2-carboxamide 5,6 Dimethoxy-1-indanone 3-Iodophenylacetic acid 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N N'Trimethyl ethylenediamine Radiator Flux Zinc Chloride Solution (All Grades) Levetiracetam Fmoc-Gln-OH Boc Phenyl Alanine Alpha Lipoic Acid*Related products of tetrahydrofuran
You may like
-
 (+)-JQ1 CAS 1268524-70-4View Details
(+)-JQ1 CAS 1268524-70-4View Details
1268524-70-4 -
![4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
3680-69-1 -
 4'-Benzyloxy-2-bromopropiophenone 98%View Details
4'-Benzyloxy-2-bromopropiophenone 98%View Details
35081-45-9 -
 53928-30-6 98%View Details
53928-30-6 98%View Details
53928-30-6 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0 -
 29676-71-9 99%View Details
29676-71-9 99%View Details
29676-71-9 -
 (R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
(R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
196601-69-1