Ammonium metavanadate
Synonym(s):Ammonium metavanadate, Ammonium vanadate;Ammonium monovanadate;Ammonium vanadate(V)
- CAS NO.:7803-55-6
- Empirical Formula: H4NO3V
- Molecular Weight: 116.98
- MDL number: MFCD00011430
- EINECS: 232-261-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:35
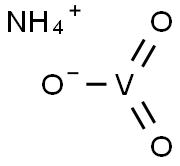
What is Ammonium metavanadate?
Chemical properties
Ammonium metavanadate is an inorganic acidic salt with the formula NH4VO3. It is a white or slightly yellow crystalline powder. Slightly soluble in cold water, hot ethanol and ether, soluble in hot water and dilute ammonium hydroxide. It is an important intermediate in the purification of vanadium.
The Uses of Ammonium metavanadate
Ammonium vanadium oxide is used as a catalyst in organic synthesis, and as an analytical reagent in laboratories. It is employed in the preparation of vanadate. It acts as a sensor for the determination of N-acetyltransferase activity in humans. Further it finds use in dyes, varnishes, indelible inks, drier for paints, and in photography. With copper carbonate, it has been shown to enhance the catalytic activities. Ammonium metavanadate is an environmental-friendly catalyst to prepare alpha-hydroxyphosphonate derivatives in high yield through the reaction of aryl/heteroaryl aldehydes with triethylphosphonate derivatives.
The Uses of Ammonium metavanadate
In dyeing and printing on woolens; staining wood black; manufacture of vanadium black and "indelible ink"; producing vanadium luster on pottery; as photographic developer; in hematoxylin staining in microscopy; as reagent in analytical chemistry.
What are the applications of Application
Ammonium metavanadate is a commonly employed vanadate preparation
What are the applications of Application
Ammonium metavanadate is a water soluble inorganic acid. It is a cheaply available, mild and efficient catalyst. Its ability to alter female reproduction has been studied in swiss albino mice.
Ammonium metavanadate may be used as a substrate in the synthesis of the following:
silver vanadate (AgVO3) nanorods
manganese vanadate nanobelts
vanadium oxide hydrate semi-microspheres
It may be used as a catalyst in the synthesis of the following:
azalactone derivatives
2,4,5-triaryl-1H-imidazoles
octahydroquinazolinone derivatives
α-hydroxyphosphonates
Preparation
Ammonium metavanadate, NH4VO3, plays an important role in the preparation of vanadium oxides and other ammonium compounds, such as NH4V3O8, (NH4)2V3O8, and NH4V4O10, which were found to possess interesting electrochemical properties.
Synthesis of ammonium metavanadate: Vanadium iron powder and charcoal powder mixed with a certain proportion of granulation for roasting, and the role of liquid alkali to generate sodium vanadate, the clear liquid after concentration, filtration, neutralization with hydrochloric acid to Ph7.5 ~ 8, filtration, filtrate heating to 70 ~ 80 ℃, stirring under the addition of ammonium chloride solution, the precipitate by centrifugal separation, washing, drying, the production of ammonium metavanadate crystals.
tailoring the size and shape-new path for ammonium metavanadate synthesis
General Description
Ammonium metavanadate appears as a white crystalline powder. Slightly soluble in water and denser than water. Decomposes at 410°F. May release toxic fumes. Moderately toxic. An irritant. Used as a dryer for paints and inks, and for dyes. Loses ammonia upon heating.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Acidic inorganic salts, such as Ammonium metavanadate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion, subcutaneous, intravenous, intratracheal, and intraperitoneal routes. Moderately toxic by skin contact. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. See also VANADIUM COMPOUNDS. When heated to decomposition it emits toxic fumes of NH3, VOx, and NOx.
Potential Exposure
It is used in the metals industry to make alloys, chemical reactions, dyes, inks, varnishes, printing, medicines, and photography.
Shipping
UN2859 Ammonium metavanadate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Wash the salt with H2O until free from Cl-ions and dry it in air. It is soluble in H2O (5.18g/100mL at 15o, 10.4g/100mL at 32o) but is more soluble in dilute NH3. It crystallises from conductivity water (20mL/g). When heated at relatively low temperature, it loses H2O and NH3 to give vanadium oxide (V2O5), and at 210o it forms lower oxides. [Baker et al. Inorg Synth III 117 1950.] Its solubility in H2O is 0.52% (15o), 1% (32o) and 1.6% (50o). After washing the technical grade salt with H2O, it had Na, Mn and U at 0.06, 0.2 and 0.1 ppm, respectively. [Brauer in Handbook of Preparative Inorganic Chem (Ed. Brauer) Academic Press Vol II p 1272-1273 1965.]
Incompatibilities
Moisture forms an acidic solution. React with bases and alkaline material: may generate heat and release flammable hydrogen gas.
Properties of Ammonium metavanadate
| Melting point: | 200 °C |
| Density | 2.32 g/cm3 at 25 °C (lit.) |
| storage temp. | Store at RT. |
| solubility | H2O: soluble |
| form | Solid |
| color | White to yellow |
| Specific Gravity | 2.33 |
| PH | 7 (5.1g/l, H2O, 20℃) |
| Odor | Odorless |
| Water Solubility | 5.1 g/L (20 ºC) |
| Merck | 14,568 |
| Exposure limits | NIOSH: Ceiling 0.05 mg/m3 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. Protect from moisture - hygroscopic. |
| CAS DataBase Reference | 7803-55-6(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium vanadate (7803-55-6) |
Safety information for Ammonium metavanadate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ammonium metavanadate
Ammonium metavanadate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Ammonium vanadium oxide CAS 7803-55-6View Details
Ammonium vanadium oxide CAS 7803-55-6View Details
7803-55-6 -
 Ammonium vanadium oxide CAS 7803-55-6View Details
Ammonium vanadium oxide CAS 7803-55-6View Details
7803-55-6 -
 Ammonium vanadium oxide CAS 7803-55-6View Details
Ammonium vanadium oxide CAS 7803-55-6View Details
7803-55-6 -
 Ammonium vanadium oxide CAS 7803-55-6View Details
Ammonium vanadium oxide CAS 7803-55-6View Details
7803-55-6 -
 Ammonium vanadium oxide CAS 7803-55-6View Details
Ammonium vanadium oxide CAS 7803-55-6View Details
7803-55-6 -
 Ammonium vanadium oxide CAS 7803-55-6View Details
Ammonium vanadium oxide CAS 7803-55-6View Details
7803-55-6 -
 AMMONIUM METAVANADATE ACSView Details
AMMONIUM METAVANADATE ACSView Details
7803-55-6 -
 Ammonium Metavanadate (CAS Number: 7803-55-6)View Details
Ammonium Metavanadate (CAS Number: 7803-55-6)View Details
7803-55-6
