Ammonium carbonate
Synonym(s):Ammonium carbonate;Carbamic acid monoammonium salt mixture with ammonium carbonate;Hartshorn salt;salt of hartshorn
- CAS NO.:506-87-6
- Empirical Formula: CH8N2O3
- Molecular Weight: 96.09
- MDL number: MFCD00010890
- EINECS: 208-058-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
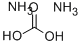
What is Ammonium carbonate?
Chemical properties
Anunonium carbonate is a white water-soluble, volatile solid prepared by reaction of NH4OH and CO2 and crystallizing from dilute alcohol. Ammonium carbonate loses NH3,CO2, and H20 at ordinary temperatures, and rapidly at 58°C.
Chemical properties
Ammonium carbonate is a colorless crystal or white lumpy powder with a strong ammonia odor. The odor specific gravity (gas)52.7; Threshold is ,5 ppm as ammonia gas.
Physical properties
Colorless or translucent hard crystalline mass or white cubic crystals or powder; sharp taste; odor of ammonia; decomposes at 58°C; slow decomposition at ambient temperatures; readily dissolves in cold water; decomposes in hot water; insoluble in liquid ammonia, alcohol and carbon disulfide.
The Uses of Ammonium carbonate
Ammonium Carbonate is a dough strengthener, a leavening agent, a ph control agent, and a texturizer. it is prepared by the sublima- tion of a mixture of ammonium sulfate and calcium carbonate, and occurs as a white powder or a hard, white translucent mass.
The Uses of Ammonium carbonate
Pharmaceutic aid (source of ammonia).
The Uses of Ammonium carbonate
Ammonium carbonate is widely utilized as a leavening agent in lebkuchen, cookies and flat biscuits. It finds an important application as an emetic and an active ingredient in some cough syrups. It is the main component in smelling salts and an active ingredient in some smokeless tobacco products, shampoos and dyes used in textile industries. It is an important predecessor to the modern leavening agent?s baking soda and baking powder. It serves as a foaming agent for the production of expanded material. It is also used as a reagent for analytical purposes in the chemical industry.
What are the applications of Application
Ammonium carbonate is a simple compound utilized as a porogen and leavening agent
Definition
A mixture of ammonium acid carbonate and ammo- nium carbamate.
Definition
ammonium carbonate: A colourlessor white crystalline solid,(NH4)2CO3, usually encountered asthe monohydrate. It is very soluble incold water. The compound decomposesslowly to give ammonia, water,and carbon dioxide. Commercial ‘ammoniumcarbonate’ is a double saltof ammonium hydrogencarbonateand ammonium aminomethanoate(carbamate), NH4HCO3.NH2COONH4.This material is manufactured byheating a mixture of ammoniumchloride and calcium carbonate andrecovering the product as a sublimedsolid. It readily releases ammoniaand is the basis of sal volatile. It isalso used in dyeing and wool preparationand in baking powders.
Preparation
Ammonium carbonate is obtained by passing carbon dioxide into aqueous ammonia solution in a column or tower. Ammonia, carbon dioxide and water vapor are distilled and the vapors condensed into a solid crystalline mass. It also may be prepared by subliming a mixture of ammonium sulfate and calcium carbonate.
General Description
A colorless crystalline solid or a white powder with a strong odor of ammonia. Noncombustible. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in pharmaceuticals, in food processing.
Air & Water Reactions
Water soluble.
Reactivity Profile
Ammonium carbonate decomposes when heated to give gaseous ammonia and gaseous carbon dioxide. Reaction is non-explosvie. Causes decomposition of sodium hypochlorite within a few seconds [Mellor 2 Supp. 1:550 1956].
Hazard
Evolves irritating fumes when heated.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion may cause gastric irritation. Contact with eyes or skin causes irritation.
Agricultural Uses
Ammonium carbonate, (NH4)2CO3, is an intermediate product formed during the synthesis of urea. Ammonium carbonate on decomposition yields urea and water.
Safety Profile
Poison by subcutaneous and intravenous routes. When heated to decomposition it emits toxic fumes of NO, and NH3.
Potential Exposure
It is used in dyeing, tanning, medicines, fire extinguishers; to make casein glue; ammonia salts; and baking powders. A laboratory reagent.
Metabolism
Not Available
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Acids, acid salts; salts of iron and zinc, alkaloids, calomel and tartar emetic. Keep cool, below 38 C. Contact with inorganic acids may form CO2, heat, and dangerous spattering.
Waste Disposal
Slowly deposit in a large container of water. Add excess amounts of soda ash and let stand for 24 hours. Decant to another container, neutralize with hydrochloric acid, and drain with an excess of water. Ship to landfill.
Properties of Ammonium carbonate
| Melting point: | 58 °C |
| Boiling point: | 179.58°C (rough estimate) |
| Density | 1.5 |
| vapor density | 2.7 (vs air) |
| vapor pressure | 760 mm Hg @ 600C |
| refractive index | 1.4616 (estimate) |
| storage temp. | Store at RT. |
| solubility | H2O: 0.1 g/mL at 25 °C, clear, colorless |
| form | Solid |
| color | White to yellow |
| Odor | strong odor of NH3 |
| PH Range | 9 |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,508 |
| BRN | 3627235 |
| CAS DataBase Reference | 506-87-6(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium carbonate (506-87-6) |
Safety information for Ammonium carbonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Ammonium carbonate
Ammonium carbonate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 AMMONIUM CARBONATE 99%View Details
AMMONIUM CARBONATE 99%View Details -
 Ammonium carbonate 99%View Details
Ammonium carbonate 99%View Details -
 Ammonium carbonate 98%View Details
Ammonium carbonate 98%View Details -
 Ammonium Carbonate CAS 506-87-6View Details
Ammonium Carbonate CAS 506-87-6View Details
506-87-6 -
 Ammonium carbonate CAS 506-87-6View Details
Ammonium carbonate CAS 506-87-6View Details
506-87-6 -
 Ammonium carbonate CAS 506-87-6View Details
Ammonium carbonate CAS 506-87-6View Details
506-87-6 -
 Ammonium carbonate CAS 506-87-6View Details
Ammonium carbonate CAS 506-87-6View Details
506-87-6 -
 Ammonium carbonate CAS 506-87-6View Details
Ammonium carbonate CAS 506-87-6View Details
506-87-6
