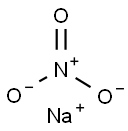
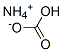
Ammonium nitrate
Synonym(s):Ammonium nitrate;Nitric acid ammonia, Ammonia nitrate
- CAS NO.:6484-52-2
- Empirical Formula: H4N2O3
- Molecular Weight: 80.04
- MDL number: MFCD00011425
- EINECS: 229-347-8
- Update Date: 2025-12-17 09:49:44

What is Ammonium nitrate?
Description
Ammonium nitrate is an oxidizer, which may explode under confinement and high temperatures. When mixed with fuel oil, a deflagrating explosive material is created. Ammonium nitrate and fuel oil were used as the explosive in the Alfred P. Murrah Federal Building terrorist attack in Oklahoma City and the first terror attack on the World Trade Center in New York City in the mid-1990s.
Chemical properties
Ammonium nitrate,NH4N03, is a colorless crystalline solid existing in two forms, Between 16 and 32°C, the crystals are tetragonal; between 32 and 84 DC, the crystals are rhombic. The melting point of NH4N03 is 169.6 DC, and it decomposes above 210°C. When heated, ammonium nitrate yields nitrous oxide gas. Ammonium nitrate is soluble in water, in acetic acid solutions containing ammonia, is slightly soluble in ethanol, and is moderately soluble in methanol.

Ammonium nitrate is a very insensitive and stable high explosive used as a slow-burning propellant for rockets when compounded with burning rate catalysts. Although the major applications of Ammonium nitrate are explosives and fertilizers, it is also used in insecticides, rust inhibitors, and pyrotechnics.
The Uses of Ammonium nitrate
Ammonium nitrate (NH4NO3, also known as “Norway saltpeter”) is mainly used as a fertilizer. It is also known as the chemical that was mixed with diesel fuel to create the explosion that demolished the Murrah Federal Building in Oklahoma City in 1995.
The Uses of Ammonium nitrate
Sold in a concentration of 27,5% N. Attributes Distributed by Agroline CH. Contact
The Uses of Ammonium nitrate
Making nitrous oxide (laughing gas); in freezing mixtures, safety explosives, matches; pyrotechnics; in fertilizers.
The Uses of Ammonium nitrate
Colorless crystals made by the action of ammonium hydroxide on nitric acid. Soluble in water, alcohol, and alkalies, ammonium nitrate is explosive and was used as a substitute for potassium nitrate in making flashlight mixtures.
Definition
ChEBI: The ammonium salt of nitric acid.
Definition
ammonium nitrate: A colourlesscrystalline solid, NH4NO3; r.d. 1.72;m.p. 169.6°C; b.p. 210°C. It is verysoluble in water and soluble inethanol. The crystals are rhombicwhen obtained below 32°C andmonoclinic above 32°C. It may bereadily prepared in the laboratory bythe reaction of nitric acid with aqueousammonia. Industrially, it is manufacturedby the same reaction usingammonia gas. Vast quantities of ammoniumnitrate are used as fertilizers(over 20 million tonnes per year)and it is also a component of someexplosives.
General Description
A colorless crystalline solid. Soluble in water. Does not readily burn but will do so if contaminated with combustible material. Accelerates the burning of combustible material. Produces toxic oxides of nitrogen during combustion. Used to make fertilizers and explosives, and as a nutrient in producing antibiotics and yeast.
Air & Water Reactions
Water soluble. Hot aqueous solutions of the nitrate above 50% conc., under confinement may decompose explosively. This process is aided catalytically with such substances as nitric acid and chloride ion, [Chem. Abs., 1982, 97, 78074].
Reactivity Profile
The hazards of AMMONIUM NITRATE have been well studied because of several extremely severe explosions [Chem. Eng., 1962, 70, 91; Bretherick, 5th Ed., 1995]. Mixtures with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. A mixture with aluminum powder (also zinc, cadmium, copper, magnesium, lead, cobalt, nickel, bismuth, chromium, and antimony) can be used as an explosive. A number of explosions in which ammonium nitrate and aluminum were mixed with carbon or hydrocarbons, with or without oxidizing agents have occurred [Mellor 5:219 1946-47]. A mixture with acetic acid ignites when warmed, especially if concentrated [Von Schwartz p. 322 1918]. Causes the decomposition of sodium hypochlorite within a few seconds [Mellor 2 Supp. 1:550 1956].
Hazard
May explode under confinement and high temperatures, but not readily detonated. Ventilate well. To fight fire, use large amounts of water. The material must be kept as cool as possible and removed from confinement and flooded with water in event of fire.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Non flammable
Agricultural Uses
Ammonium nitrate (NH4NO3) is among the most
common nitrogenous fertilizers, with half of its nitrogen
in an ammoniacal form and the rest in nitrate form.
The nitrogen from ammonium nitrate is immediately
available to plants, whereas the ammoniacal nitrogen
becomes available only after its nitration. Fertilizer grade
ammonium nitrate solution in water containing 20%
nitrogen is sold in large quantities, because of its high
water solubility and easy soil applicability. The fertilizer
can also be used in its compound forms, such as calcium
ammonium nitrate and ammonium sulphate nitrate.
The nitrogen in ammonium nitrate is more rapidly
available than that in urea or ammonium sulphate. Crops
take up nitrogen mainly in the form of nitrate. The
ammoniacal nitrogen must be converted to nitrate in the
soil before it becomes effective. Urea causes seedling
damage due to volatilization of ammonia. Ammonium
nitrate and ammonium sulphate are strongly acidforming
fertilizers.
Although pure salt ammonium nitrate is a fine white
crystal, it is usually available as white granules or prills
of 1.2 to 3.3 mm size and contains 32 to 35 % nitrogen by
weight. The crystalline form is highly hygroscopic and is
readily soluble in water. Because of this high solubility in
water, it is less effective for flooded rice than urea or
other ammoniacal nitrogen fertilizers. It is also more
prone to leaching than other ammoniacal products. It is
also a very powerful oxidizing agent which can explode
when exposed to heat or flame. Because of this hazardous
property, ammonium nitrate and its compounds are
stored in a dry place in sealed bags. The granular form,
however, is easily stored. It can also be spread on soil
with ease.
Ammonium nitrate, like other ammonium
fertilizers, can leave behind an acid residue in the soil. It
takes 0.8 kg of lime to neutralize the acidity produced by
1 kg of ammonium nitrate fertilizer.
Ammonium nitrate and urea are the most widely used
sources of nitrogen from among all solid fertilizers
available. A two bale crop of cotton removes 56 kg of
nitrogen per hectare in the seed alone and 16.8 kg of
nitrogen per hectare in the lint. To supply this amount of
nitrogen, the addition of 224 kg per hectare of ammonium
nitrate is required. A 2240 kg per hectare tobacco crop
removes 123 kg of nitrogen per hectare, and 5600 kg per
hectare crop of rice removes 90 kg of nitrogen per
hectare.
Ammonium nitrate is used as a major source of
nitrogen for crops in the USA. It is a good nitrogen plant
food for all field and vegetable crops and can be applied
to the soil before or at the time of planting. It also makes
for a good N-fertilizer for side dressing or top dressing.
An aqueous solution of urea and ammonium nitrate,
called UAN is used as a liquid nitrogen fertilizer.
‘Ammonite’ is a trademark for a mixture of ammonium
nitrate (98%) and coating agents (2%).
Safety Profile
A powerful oxidizer and an allergen. See also NITRATES. A relatively stable explosive that has, however, caused many industrial explosions. Violent or explosive spontaneous reactions with acetic anhydride + nitric acid, ammonium sulfate + potassium, copper iron(Ⅱ) sulfide, sawdust, urea, barium nitrate, hot water, and ammonium chloride + water + zinc. Forms heator shock-sensitive explosive mixtures with acetic acid, aluminum + calcium nitrate + formamide (a blasting explosive), ammonia, charcoal + metal oxides (e.g., rust, copper oxide, zinc oxide above 80℃), chloride salts (e.g., ammonium chloride, calcium chloride, iron(ⅡI) chloride, and aluminum chloride), cyanoguanidine, feruH2ers (e.g., super phosphate + organic materials above 90℃), hydrocarbon oils, powdered metals (e.g., aluminum, antimony, bismuth, cadmium, chromium, cobalt, copper, iron, lead, magnesium, manganese, nickel, tin, zinc, brass, stainless steel, titanium, and potassium), nonmetals (e.g., charcoal, and phosphorus), organic fuels (e.g., wax, oils, and stearates), potassium permanganate, sugar, sulfur, and trinitroanisole. Reaction with alkali metals (e.g., sodium) forms an explosive product. Ignites on contact with ammonium dichromate, potassium dichromate, potassium chromate, barium chloride, sodium chloride, potassium nitrate, and chromium(V1) salts. Can ignite when mixed with acetic acid. Use water in large amounts to fight fire. It is important that the mass of materials be kept cool and that burning be extinguished promptly. Ventilate well. May explode under confinement and high temperatures. When heated to decomposition it emits highly toxic fumes of NOx. Can react vigorously with reducing materials. Incompatible with, (NH4Cl + heat), (C + heat), organic matter, P, NaOCl, NaClO4. Occasional explosions in presence of oil, (NH4)2S04 with K or Na.
Potential Exposure
Used in the manufacture of liquid and solid fertilizer compositions, industrial explosives and blasting agents from ammonium nitrate, matches; antibiotics; in the production of nitrous oxide.
Metabolism
Ammonium Nitrate. Ammonium nitrate is one of the two leading nitrogen fertilizer materials on a world basis: 10% in 1997. The high N content is advantageous for the reduction of freight and application costs per unit weight of nitrogen. The presence of 50% of the nitrogen in the highly available nitrate form makes it suitable for use in regions growing crops with a short vegetation period but has the disadvantage that, because the NO3 ? ion is not adsorbed by soil, it may contribute to relatively large nitrogen losses by the leaching of increased soil nitrate into streams and groundwater. Although the application of any nitrogenous fertilizer results in some degree of soil acidification, the nitrate form is notably less acidifying than ammonium sulfate and has a lower tendency for the loss of nitrogen to the atmosphere as gaseous ammonia. The hygroscopic character of the crystalline material, coupled with its explosive nature, contributes to difficult storage and handling properties and the need for the production of purified and stabilized forms.
Shipping
Ammonium nitrate with organic coating: UN0222 Ammonium nitrate, with . 0.2% combustible substances, including any organic substance calculated as carbon, to the exclusion of any other added substance, Hazard Class: 1D; Labels:1D-Explosive (with a mass explosion hazard); D-Substances or articles which may mass detonate (with blast and/or fragment hazard) when exposed to fire. Ammonium nitrate with NO organic coating: UN1942 Ammonium nitrate, with NOT . 0.2% of combustible substances, including any organic substance calculated as carbon, to the exclusion of any other added substance (also used for fertilizer), Hazard Class: 5.1; Labels: 5.1-Oxidizer. UN3375 Ammonium nitrate emulsion or Ammonium nitrate suspension or Ammonium nitrate gel, intermediate for blasting explosives, Hazard Class: 5.1; Labels: 5.1-Oxidizer. UN2072 Ammonium nitrate fertilizer, n.o.s., doesn’t appear in the 49 CFR Hazmat Table, refer to UN1942, above). UN2071 Ammonium nitrate based fertilizer, Hazard class: 9; Labels: 9-Miscellaneous hazardous material. UN2426/140 Ammonium nitrate, liquid (hot concentrated solution), Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
It is crystallised twice from distilled water (1mL/g) by adding EtOH, or from warm water (0.5mL/g) by cooling in an ice-salt bath. Dry it in air, then under vacuum. After 3 recrystallisations of ACS grade, it contained Li and B at 0.03 and 0.74 ppm, respectively. It is deliquescent. [Early & Lowry J Chem Soc 115 1387 1919, 121 963 1922, Hendricks et al. J Am Chem Soc 54 2766 1932.]
Incompatibilities
A strong oxidizer. Reducing agents; combustible materials; organic materials; finely divided (powdered) metals may form explosive mixtures or cause fire and explosions. When contaminated with oil, charcoal or flammable liquids, can be considered an explosive which can be detonated by combustion or shock.
Waste Disposal
Pretreatment involves addition of sodium hydroxide to liberate ammonia and form the soluble sodium salt. The liberated ammonia can be recovered and sold. After dilution to the permitted provisional limit, the sodium salt can be discharged into a stream or sewer.
Properties of Ammonium nitrate
| Melting point: | 169 °C(lit.) |
| Boiling point: | 210 °C(lit.) |
| Density | 1.72 |
| Flash point: | 210°C |
| storage temp. | Store at RT. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| color | White |
| Specific Gravity | 1.725 |
| PH | 4.5-6.0 (25℃, 50mg/mL in H2O) |
| Water Solubility | 190 g/100 mL (20 ºC) |
| Merck | 14,534 |
| CAS DataBase Reference | 6484-52-2(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium nitrate (6484-52-2) |
Safety information for Ammonium nitrate
| Signal word | Warning |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Ammonium nitrate
Ammonium nitrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
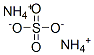
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
