Ammonium acetate
Synonym(s):Acetic Acid, Ammonium Salt;Ammonium acetate;Ammonium ethanoate;buffers
- CAS NO.:631-61-8
- Empirical Formula: C2H7NO2
- Molecular Weight: 77.08
- MDL number: MFCD00013066
- EINECS: 211-162-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:32
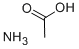
What is Ammonium acetate?
Description
Ammonium acetate (NH4OAc) is a commonly used ammonium salt that can be produced by the reaction of ammonia and acetic acid. NH4OAc is mostly used as a buffer solution, buffering near pH 4.75 (pKa for acetic acid) and pH 9.25 (pKa for ammonium). NH4OAc is also used in the Henley reaction.
Chemical properties
Ammonium acetate is a white, deliquescent crystalline solid with a slightly acetic odor. It is used as a reagent in analytical chemistry for determining lead and iron content,a chemical intermediate in manufacturing acetamide. It is also a diuretic in veterinary medicine, in other drugs, in textile dyeing, in meat preservative, in foam rubbers, in vinyl plastics, in stripping explosives, and in determining lead and iron content.

Physical properties
White crystalline solid; deliquescent; melts at 114°C; decomposes at elevated temperatures; density 1.17 g/cm3 at 20°C, density of a 10% solution 1.022 g/mL, and a 50% solution 1.092 g/mL; very soluble in cold water (1,480 g/L at 4°C); also soluble in cold alcohol and acetone (78.9 g/L in methanol at 15°C); solution loses ammonia on standing and becomes acidic.
The Uses of Ammonium acetate
Ammonium acetate is used in the manufacture of acetamide and as a diuretic and diaphoretic in medical applications. The wool industry also uses this salt as a dye mordant.
Buffer solution; determination of lead and iron; separating lead sulfate from other sulfates.
The Uses of Ammonium acetate

NH4OAc (316 mg, 4.10 mmol) was added to a solution of the SM (2.00 g, 6.84 mmol) in nitromethane (517 mL). The reaction mixture was stirred at 60 C for 18 h. The mixture was quenched with H2O and DCM was added. The layers were separated and the org layer was washed with brine, dried (MgSO4), and concentrated to give an orange oil. The material (1.8 g) was purified by Prep LC (50 g silica, 0-5% EtOAc/heptane) to provide the product as a yellow oil. [750 mg, 33%]
The Uses of Ammonium acetate
Ammonium acetate solution can be used to study molecular biology, biological buffers, reagents and DNA and RNA purification. Ammonium acetate solution has been used to study pharmacokinetic analysis of α and β epimers of glycyrrhetinic acid in rat plasma. Ammonium acetate solution has also been used in a study to develop a method for the simultaneous determination of aristolochic acids A and B in some Chinese herbals and traditional Chinese patent medicines.
Preparation
Ammonium acetate is manufactured by neutralizing acetic acid with ammonium carbonate or by passing ammonia gas into glacial acetic acid. Acidic ammonium acetate, CH3CO2NH4.CH3CO2H[25007-86-7], is manufactured by dissolving the neutral salt in acetic acid.
Definition
ChEBI: Ammonium acetate is an ammonium salt obtained by reaction of ammonia with acetic acid. A deliquescent white crystalline solid, it has a relatively low melting point (114℃) for a salt. Used as a food acidity regulator, although no longer approved for this purpose in the EU. It has a role as a food acidity regulator and a buffer. It is an acetate salt and an ammonium salt.
General Description
A white crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium acetate is used in chemical analysis, in pharmaceuticals, in preserving foods, and for other uses.
Reactivity Profile
Ammonium acetate causes the decomposition of sodium hypochlorite within a few seconds [Mellor 2 Supp. 1:550 1956].
Health Hazard
Inhalation of dust irritates nose and mouth. Ingestion irritates mouth and stomach. Contact with dust causes irritation of eyes and mild irritation of skin.
Fire Hazard
Special Hazards of Combustion Products: Irritating vapors of ammonia acetic acid, and nitrogen oxides may form in fires.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Ammonium acetate can be used in proteinaceous baits and in beer waste to increase their attraction to Ceratitis capitate. It helps in the progression of acetone-butanol-ethanol (ABE) fermentation when it is used as the only nitrogen source.
Safety Profile
Poison by intravenous route. Moderately toxic by intraperitoneal route. When heated to decomposition it emits toxic fumes of NO, and NH3
Potential Exposure
Ammonium acetate is used as a chemical reagent, to make drugs; foam rubber; vinyl plastics; explosives, and to preserve foods. An environmental threat.
Ammonium acetate Application
Ammonium acetate is a reagent used in chromatographic analysis of various compounds such as oligos, proteins, and peptides. It is also employed with acetic acid to provide a buffer solution. Generally it behaves as a catalyst in many reactions like Knoevenagel, Borch. It is also employed as a nutrient additive.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Combustible solid. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, sodium hypochlorite, potassium chlorate, sodium nitrite.
Properties of Ammonium acetate
| Melting point: | 110-112 °C (dec.) (lit.) |
| Boiling point: | 138.46°C (rough estimate) |
| Density | 1.07 g/mL at 20 °C |
| vapor pressure | 0.017-0.02Pa at 25℃ |
| refractive index | 1.4350 (estimate) |
| Flash point: | 136 °C |
| storage temp. | Store at +15°C to +25°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| appearance | White solid |
| pka | 4.6(Acetic Acid), 9.3(Ammonium Hydroxide)(at 25℃) |
| color | White |
| Odor | Slight acetic acid odor |
| PH | 7(1 mM solution);7(10 mM solution);7(100 mM solution);6.95(1000 mM solution) |
| PH Range | 6.7 - 7.3 |
| Water Solubility | 1480 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.015 λ: 280 nm Amax: 0.01 |
| Merck | 14,495 |
| BRN | 4186741 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 631-61-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Ammonium acetate, anhydrous(631-61-8) |
| EPA Substance Registry System | Ammonium acetate (631-61-8) |
Safety information for Ammonium acetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ammonium acetate
| InChIKey | USFZMSVCRYTOJT-UHFFFAOYSA-N |
Ammonium acetate manufacturer
VJ CHEMTRADE PVT LTD
Uma Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 AMMONIUM ACETATE 99% 99%View Details
AMMONIUM ACETATE 99% 99%View Details -
 Ammonium acetate 99%View Details
Ammonium acetate 99%View Details -
 Ammonium acetate 99%View Details
Ammonium acetate 99%View Details -
 Ammonium acetate 98%View Details
Ammonium acetate 98%View Details -
 Ammonium acetate CAS 631-61-8View Details
Ammonium acetate CAS 631-61-8View Details
631-61-8 -
 Ammonium acetate CAS 631-61-8View Details
Ammonium acetate CAS 631-61-8View Details
631-61-8 -
 Ammonium Acetate, 97% 99%View Details
Ammonium Acetate, 97% 99%View Details -
 Ammonium AcetateView Details
Ammonium AcetateView Details
631-61-8
