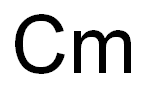americium
- CAS NO.:7440-35-9
- Empirical Formula: C5H12
- Molecular Weight: 72.14878
- EINECS: 231-144-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
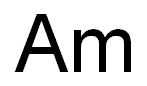
What is americium ?
Chemical properties
silvery metal with two forms; α-Am: hexagonal, a=0.3468 nm, c=1.1241 nm; β-Am: cub, a=0.4894 nm; t1/2 241Am=433 years, t1/2242Am=152 h, t1/2 243Am=7400 years; Am+++ stable in aqueous solution; enthalpy of vaporization 230 kJ/mol; enthalpy of fusion 14.4 kJ/mol; enthalpy of sublimation 276 kJ/mol; ionic radius of Am+++ is 0.0982nm; can be prepared from 241Pu; used to diagnose thyroid disorders [KIR78] [CIC73]
Chemical properties
AMERICIUM, Chemical element, symbol Am, at no. 95, at. wt. 243 (mass number of the most stable isotope), radioactive metal of the actinide series, also one of the transuranium elements. All isotopes of americium are radioactive; all must be produced synthetically. The element was discovered by G.T. Seaborg and associates at the Metallurgical Laboratory of the University of Chicago in 1945. At that time, the element was obtained by bombarding uranium-238 with helium ions to produce 241Am, which has a half-life of 475 years. Subsequently, 241Am has been produced by bombardment of plutonium-241 with neutrons in a nuclear reactor. 243Am is the most stable isotope, an alpha emitter with a half-life of 7950 years. Other known isotopes are 237Am,238 Am,240 Am,241Am,242Am,244 Am,245 Am, and 246Am.
This element exists in acidic aqueous solution in the (III), (IV), (V), and (VI) oxidation states with the ionic species probably corresponding to Am3+, Am4+, AmO2 + and AmO2 2+. The colors of the ions are: Am3+, pink; Am4+, rose; AmO2 +, yellow; and AmO2 2+, rum-colored.
Physical properties
All the isotopes of americium belonging to the transuranic subseries of the actinide seriesare radioactive and are artificially produced. Americium has similar chemical and physicalcharacteristics and is homologous to europium, located just above it in the rare-earth (lanthanide)series on the periodic table. It is a bright-white malleable heavy metal that is somewhatsimilar to lead. Americium’s melting point is 1,176°C, its boiling point is 2,607°C, and itsdensity is 13.68g/cm3.
Isotopes
There are 24 isotopes of americium. All are radioactive with half-lives rangingfrom 72 microseconds to over 7,000 years. Five of americium’s isotopes are fissionablewith spontaneous alpha decay.
Origin of Name
Named after the continent America because Europium was named after the European continent.
Occurrence
Americium does not exist in nature. All of its isotopes are man-made and radioactive.Americium-241 is produced by bombarding plutonium-239 with high-energy neutrons,resulting in the isotope plutonium-240 that again is bombarded with neutrons and resultsin the formation of plutonium-241, which in turn finally decays into americium-241 by theprocess of beta decay. Both americium-241 and americium-243 are produced within nuclearreactors. The reaction is as follows: 239Pu + (neutron and λ gamma rays) → 240Pu + (neutronand λ gamma rays) → 241Pu→ 241Am + beta minus (β-); followed by 241Am→ 93Np-237 +4He2 (helium nuclei).
History
Americium was the fourth transuranium element to be discovered; the isotope 241 Am was identified by Seaborg, James, Morgan, and Ghiorso late in 1944 at the wartime Metallurgical Laboratory of the University of Chicago as the result of successive neutron capture reactions by plutonium isotopes in a nuclear reactor: 239Pu(n,γ)→240Pu(n,γ)→241Pu?β →83Am Since the isotope 241Am can be prepared in relatively pure form by extraction as a decay product over a period of years from strongly neutron-bombarded plutonium, 241Pu, this isotope is used for much of the chemical investigation of this element. Better suited is the isotope 243Am due to its longer half-life (7.37 × 103 years as compared to 432.2 years for 241Am). A mixture of the isotopes 241Am, 242Am, and 243Am can be prepared by intense neutron irradiation of 241Am according to the reactions 241Am (n, γ) → 242Am (n, γ) → 243Am. Nearly isotopically pure, 243Am can be prepared by a sequence of neutron bombardments and chemical separations as follows: neutron bombardment of 241Am yields 242Pu by the reactions 241Am (n, γ) → 242Am → 242Pu, after chemical separation the 242Pu can be transformed to 243Am via the reactions 242Pu (n, γ) → 243Pu → 243Am, and the 243Am can be chemically separated. Fairly pure 242Pu can be prepared more simply by very intense neutron irradiation of 239Pu as the result of successive neutroncapture reactions. Seventeen radioactive isotopes and isomers are now recognized. Americium metal has been prepared by reducing the trifluoride with barium vapor at 1000 to 1200°C or the dioxide by lanthanum metal. The luster of freshly prepared americium metal is white and more silvery than plutonium or neptunium prepared in the same manner. It appears to be more malleable than uranium or neptunium and tarnishes slowly in dry air at room temperature. Americium is thought to exist in two forms: an alpha form which has a double hexagonal close-packed structure and a loose-packed cubic beta form. Americium must be handled with great care to avoid personal contamination. As little as 0.03 μCi of 241Am is the maximum permissible total body burden. The alpha activity from 241Am is about three times that of radium. When gram quantities of 241Am are handled, the intense gamma activity makes exposure a serious problem. Americium dioxide, AmO2, is the most important oxide. AmF3, AmF4, AmCl3, AmBr3, AmI3, and other compounds have been prepared. The isotope 241Am has been used as a portable source for gamma radiography. It has also been used as a radioactive glass thickness gage for the flat glass industry, and as a source of ionization for smoke detectors. Americum-243 (99%) is available from the Oak Ridge National Laboratory at a cost of about $750/g plus packing charges.
Characteristics
Although americium’s main valence (oxidation state) is +3, it is tetravalent. It can formcompounds with its ions of +4, +5, and +6, particularly when oxidized. Its most stable isotopeis americium-243, with a half-life of 7,379 years, which, over time through alpha decay,transmutates into neptunium-239.
The Uses of americium
Gamma radiography, radiochemical research, diagnostic aid, electronic devices.
The Uses of americium
Americium-141, with a half-life of 432 years, which produces both alpha (helium nuclei)and gamma rays (somewhat like X-rays), has found some commercial uses. One use is as thedetector used in household smoke alarms The americium-141 isotope emits alpha particlesthat ionize air by removing electrons from the molecules of air and improving its electricalconductivity. Smoke reduces this conductivity, thus triggering the alarm. Another use is americium-241’s high-energy gamma rays in radiography, in which portable X-ray instruments caneasily be transported for emergency use. As a gamma ray source, americium-241 is used as adiagnostic aid to check the quality of welds in metals.
Definition
Am. A synthetic radioactive element of atomic number 95, a member of the actinide series; aw 241; 14 isotopes of widely varying half-life; valence 3, but divalent, tetravalent, and higher valencies exist. α and γ emitter, forms compounds with oxygen, halides, lithium, etc. Metallic americium is silver-white crystalline, d 13.6, mp approximately 100C. Half-life of 241 Am is 458 years.
Definition
A highly
toxic radioactive silvery element of the
actinoid series of metals. A transuranic element,
it is not found naturally on Earth but
is synthesized from plutonium. The element
can be obtained by reducing the trifluoride
with barium metal. It reacts with
oxygen, steam, and acids. 241Am has been
used in gamma-ray radiography.
Symbol: Am; m.p. 1172°C; b.p.
2607°C; r.d. 13.67 (20°C); p.n. 95; most
stable isotope 243Am (half-life 7.37 × 103
years).
Definition
americium: Symbol Am. A radioactivemetallic transuranic elementbelonging to the actinoids; a.n. 95;mass number of most stable isotope243 (half-life 7.95 × 103 years); r.d.13.67 (20°C); m.p. 994 ± 4°C; b.p.2607°C. Ten isotopes are known. Theelement was discovered by G. T.Seaborg and associates in 1945, whoobtained it by bombarding uranium–238 with alpha particles.
Hazard
A radioactive poison.
Hazard
All the isotopes and compounds of americium are deadly sources of radiation and causeradiation poisoning and death. Precautions must be taken when working with it. The smallamount of americium-241 found in smoke detectors in household smoke alarms is harmlessunless the isotope is removed and swallowed.
Properties of americium
| Melting point: | 1175℃ |
| Boiling point: | bp (calc) 2067° |
| Density | 13.671 |
| solubility | soluble in acid solutions |
| form | silvery metal |
| color | silvery metal; hexagonal, hexane or
cubic |
| Water Solubility | soluble dilute acids [CRC10] |
| EPA Substance Registry System | Americium (7440-35-9) |
Safety information for americium
Computed Descriptors for americium
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8