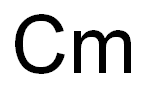plutonium
- Empirical Formula: Pu
- Molecular Weight: 244
- EINECS: 231-117-7

What is plutonium ?
Physical properties
All isotopes of plutonium are radioactive. The two isotopes that have found the most usesare Pu-238 and Pu-239. Pu-238 is produced by bombarding U-238 with deuterons in acyclotron, creating neptunium-238 and two free neutrons. Np-238 has a half-life of abouttwo days, and through beta decay it transmutates into plutonium-238. There are six allotropicmetallic crystal forms of plutonium. They all have differing chemical and physical properties.The alpha (α) allotrope is the only one that exists at normal room temperatures and pressures.The alpha allotrope of metallic plutonium is a silvery color that becomes yellowish as it oxidizesin air. All the other allotropic forms exist at high temperatures.
The most stable isotope of plutonium is Pu-244, with a half-life of 8.00×10+7years (about82,000,000 years). Being radioactive, Pu-244 can decay in two different ways. One wayinvolves alpha decay, resulting in the formation of the isotope uranium-240, and the other isthrough spontaneous fission.
The melting point of plutonium is 640°C, its boiling point is 3,232°C, and its density isover 19 times that of the same volume of water (19.84g/cm3).
Isotopes
There are a total of 24 isotopes of plutonium. All of them are unstable andradioactive. Their half-lives range from 28 nanoseconds to 8.00×10+7years.
Origin of Name
Named for the planet Pluto.
Occurrence
Plutonium exists in trace amounts in nature. Most of it isotopes are radioactive and manmadeor produced by the natural decay of uranium. Plutonium-239 is produced in nuclearreactors by bombarding uranium-238 with deuterons (nuclei of deuterium, or heavy hydrogen).The transmutation process is as follows: 238U + deuterons→ 2 nuclei + 239Np + β→decays to→ 238Pu + β-.
There is more than an adequate supply of plutonium-239 in the world because it is a“waste” product of the generation of electricity in nuclear power plants. One of the objectionsto developing more nuclear reactors is the dilemma of either eliminating or storing allthe excess plutonium. In addition, there is always the risk of terrorists’ obtaining a supply ofPu-239 to make nuclear weapons.
History
Plutonium was the second transuranium
element of the actinide series to be discovered. The isotope
238Pu was produced in 1940 by Seaborg, McMillan, Kennedy,
and Wahl by deuteron bombardment of uranium in the 60-
inch cyclotron at Berkeley, California. Plutonium also exists in trace quantities in naturally occurring uranium ores. It is
formed in much the same manner as neptunium, by irradiation
of natural uranium with the neutrons that are present. By
far of greatest importance is the isotope Pu239, with a half-life
of 24,100 years, produced in extensive quantities in nuclear
reactors from natural uranium.
Nineteen isotopes of plutonium are now known.
Plutonium has assumed the position of dominant importance
among the transuranium elements because of its successful use
as an explosive ingredient in nuclear weapons and the place it
holds as a key material in the development of industrial use of
nuclear power. One kilogram is equivalent to about 22 million
kilowatt hours of heat energy. The complete detonation of a
kilogram of plutonium produces an explosion equal to about
20,000 tons of chemical explosive. Its importance depends on
the nuclear property of being readily fissionable with neutrons
and its availability in quantity. The world’s nuclear-power reactors
are now producing about 20,000 kg of plutonium/yr.
By 1982 it was estimated that about 300,000 kg had accumulated.
The various nuclear applications of plutonium are well
known. 238Pu has been used in the Apollo lunar missions to
power seismic and other equipment on the lunar surface. As
with neptunium and uranium, plutonium metal can be prepared
by reduction of the trifluoride with alkaline-earth metals.
The metal has a silvery appearance and takes on a yellow
tarnish when slightly oxidized. It is chemically reactive. A relatively
large piece of plutonium is warm to the touch because
of the energy given off in alpha decay. Larger pieces will produce
enough heat to boil water. The metal readily dissolves in
concentrated hydrochloric acid, hydroiodic acid, or perchloric
acid with formation of the Pu+3 ion. The metal exhibits six allotropic
modifications having various crystalline structures. The
densities of these vary from 16.00 to 19.86 g/cm3. Plutonium
also exhibits four ionic valence states in aqueous solutions:
Pu+3(blue lavender), Pu+4 (yellow brown), PuO+ (pink?), and
PuO+2 (pink orange). The ion PuO+ is unstable in aqueous solutions,
disproportionating into Pu+4 and PuO+2. The Pu+4 thus
formed, however, oxidizes the PuO+ into PuO+2, itself being reduced
to Pu+3, giving finally Pu+3 and PuO+2. Plutonium forms
binary compounds with oxygen: PuO, PuO2, and intermediate
oxides of variable composition; with the halides: PuF3, PuF4,
PuCl3, PuBr3, PuI3; with carbon, nitrogen, and silicon: PuC,
PuN, PuSi2. Oxyhalides are also well known: PuOCl, PuOBr,
PuOI. Because of the high rate of emission of alpha particles
and the element being specifically absorbed by bone marrow,
plutonium, as well as all of the other transuranium elements
except neptunium, are radiological poisons and must be handled
with very special equipment and precautions. Plutonium
is a very dangerous radiological hazard. Precautions must also
be taken to prevent the unintentional formation of a critical
mass. Plutonium in liquid solution is more likely to become
critical than solid plutonium. The shape of the mass must also
be considered where criticality is concerned. Plutonium-239
is available to authorized users from the O.R.N.L. at a cost of
about $4.80/mg (99.9%) plus packing costs.
Characteristics
A single kilogram of radioactive metallic plutonium-238 produces as much as 22million kilowatt-hours of heat energy. Larger amounts of Pu-238 produce more heat.However, Pu-238 is not fissionable, and thus it cannot sustain a chain reaction. However,plutonium-239 is fissionable, and a 10-pound ball can reach a critical mass sufficient tosustain a fission chain reaction, resulting in an explosion, releasing the equivalent of over20,000 tons of TNT. This 10-pound ball of Pu-239 is only about one-third the size offissionable uranium-235 required to reach a critical mass. This makes plutonium-239 thepreferred fissionable material for nuclear weapons and some nuclear reactors that produceelectricity.
Plutonium has some peculiar qualities. In its molten state it will corrode any containerin which it is stored. It has an unusual ability to combine with almost all the other elementslisted on the periodic table, and it can change its density by as much as 25% according toits environment. Small pieces of it can spontaneously ignite at temperatures as low as 150°C.A modern atomic bomb has three main parts: the primary, the secondary, and a radiationcontainer. The primary is formed as a small ball of plutonium called a “pit,” which is used forthe “core” of an atomic (nuclear) bomb. This pit is surrounded by the secondary component,which is a chemical explosive material, which is then surrounded by a shell of uranium. Whenthe chemical explosive is triggered, it implodes, compressing the plutonium core, which resultsin an increase in the pit’s density. This forces the nuclei of the atoms closer together, producing“free” neutrons. This compression causes the plutonium nuclei to fission in a chain reactionthat results in the release of tremendous energy. This reaction also causes the surroundinguranium to fission, which releases more nuclear energy—enough to wipe out New York Cityor Washington, D.C.
The first nuclear bomb that was tested was called “Trinity” and weighed 10,000 pounds.Today’s bombs weigh about 250 pounds and are about the size of an average suitcase.
The Uses of plutonium
The most common use of plutonium is as a fuel in nuclear reactors to produce electricity oras a source for the critical mass required to sustain a fission chain reaction to produce nuclearweapons. Plutonium also is used to convert nonfissionable uranium-238 into the isotopecapable of sustaining a controlled nuclear chain reaction in nuclear power plants. It takesonly 10 pounds of plutonium-239 to reach a critical mass and cause a nuclear explosion, ascompared with about 33 pounds of fissionable, but scarce, uranium-235.
Plutonium-238 and plutonium-239 are two isotopes that can be used outside of thenuclear weapons industry. Plutonum-238 is currently used in small thermoelectric generatorsto provide electricity for space probes that are sent far beyond the region where the sun couldbe used to generate electric power. Two early instruments sent beyond our solar system arethe Galileo and Cassini probes. Plutonium-239’s critical mass undergoes a fissionable chainreaction, making it ideal for use as fuel for some types of nuclear reactors as well as to producenuclear weapons. In the future it may be possible to use all the waste plutonium produced inthe world to power small thermal electrical power plants that could be installed in each houseto provide inexpensive and continuous household electrical power. This probably will nothappen until the public overcomes its fear of nuclear energy.
Definition
A radioactive silvery element of the actinoid series of metals. It is a transuranic element found on Earth only in minute quantities in uranium ores but readily obtained, as 239Pu, by neutron bombardment of natural uranium. The readily fissionable 239Pu is a major nuclear fuel and nuclear explosive. Plutonium is highly toxic because of its radioactivity; in the body it accumulates in bone. Symbol: Pu; m.p. 641°C; b.p. 3232°C; r.d. 19.84 (25°C); p.n. 94; most stable isotope 244Pu (half-life 8.2 × 107 years).
Definition
plutonium: Symbol Pu. A dense silveryradioactive metallic transuranicelement belonging to the actinoids;a.n. 94; mass number of most stableisotope 244 (half-life 7.6×107 years);r.d. 19.84; m.p. 641°C; b.p. 3232°C.Thirteen isotopes are known, by farthe most important being plutonium–239 (half-life 2.44×104 years),which undergoes nuclear fission withslow neutrons and is therefore a vitalpower source for nuclear weaponsand some nuclear reactors. About 20tonnes of plutonium are producedannually by the world’s nuclear reactors.The element was first producedby Seaborg, McMillan, Kennedy, andWahl in 1940.
Hazard
Plutonium is by far one of the most toxic radioactive poisons known. The metal, its alloys,and its compounds must be handled in a shielded and enclosed “glove box” that contains an inertargon atmosphere. It is a carcinogen that can cause radiation poisoning leading to death.
Industrial uses
Plutonium is made from uranium-238 byabsorption of neutrons from recycled fuel. Themetal, 99.8% pure, is obtained by reduction ofplutonium fluoride, PuF4, or plutonium chloride,PuCl3. Plutonium-238 has a low radiationlevel and is used as a heat source for smallwater-circulating heat exchangers for navalundersea diving suits.
Plutonium-241 emits beta and gamma rays.Because all the allotropic forms are radioactive,it is a pure nuclear fuel in contrast touranium, which is only 0.7% directly usefulfor fission. It is thus necessary to dilute plutoniumfor control. For fuel elements it may bedispersed in stainless steel and pressed intopellets at about 871°C, or pellets may be madeof plutonium carbide. Plutonium–iron alloy,with 9.5% iron, melts at 410°C. It is encasedin a tantalum tube for use as a reactor fuel.Plutonium–aluminum alloy is also used.
Safety information for plutonium
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8