curium
- CAS NO.:7440-51-9
- Empirical Formula: Cm
- Molecular Weight: 247
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
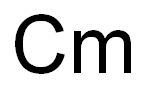
What is curium?
Chemical properties
silvery white, hard brittle metal; chemistry of trivalent state similar to that of trivalent lanthanides; α-emitter; hexagonal, a=0.3496nm, c=1.1331 nm; enthalpy of vaporization 1340 kJ/mol; ionic radius of Cm+++ is 0.0970nm; discovered in 1944; used in generating thermoelectric power for remote locations and in space; β-Cm is fcc, which is stable at <1340°C [HAW93] [MER06] [KIR78]
Physical properties
After the discovery of plutonium and before elements 95 and 96 were discovered, theirexistence and properties were predicted. Additionally, chemical and physical properties werepredicted to be homologous (similar) to europium (63Eu) and gadolinium (64Gd), locatedin the rare-earth lanthanide series just above americium (95Am) and curium (96Cm) on theperiodic table. Once discovered, it was determined that curium is a silvery-white, heavymetal that is chemically more reactive than americium with properties similar to uraniumand plutonium. Its melting point is 1,345°C, its boiling point is ~1,300°C, and its density is13.51g/cm3.
Isotopes
There are 23 isotopes of curium. All of them are man-made and radioactive.The most stable is curium-247, with a half-life of 1.56×10+7years (156,600,000 years),which through alpha decay transmutates into plutonium-243.
Origin of Name
Named after Pierre and Marie Curie.
Occurrence
There is no natural curium on Earth. All of its isotopes are man-made and artificiallyproduced through nuclear reactions with other elements. The curium isotope Cm-242 wasfirst produced by bombarding plutonium-239 with helium nuclei (alpha particles), whichcontributed neutrons that changed 94Pu to 96Cm.
History
Although curium follows americium in the periodic system, it was actually known before americium and was the third transuranium element to be discovered. Curium was identified by Seaborg, James, 4-10 The Elements and Ghiorso in 1944 at the wartime Metallurgical Laboratory in Chicago as a result of helium-ion bombardment of 239Pu in the Berkeley, California, 60-inch cyclotron. Visible amounts (30 μg) of 242Cm, in the form of the hydroxide, were first isolated by Werner and Perlman of the University of California in 1947. In 1950, Crane, Wallmann, and Cunningham found that the magnetic susceptibility of microgram samples of CmF3 was of the same magnitude as that of GdF3. This provided direct experimental evidence for assigning an electronic configuration to Cm+3. In 1951, the same workers prepared curium in its elemental form for the first time. Sixteen isotopes of curium are now known. The most stable, 247Cm, with a half-life of 16 million years, is so short compared to the Earth’s age that any primordial curium must have disappeared long ago from the natural scene. Minute amounts of curium probably exist in natural deposits of uranium, as a result of a sequence of neutron captures and β decays sustained by the very low flux of neutrons naturally present in uranium ores. The presence of natural curium, however, has never been detected. 242Cm and 244Cm are available in multigram quantities. 248Cm has been produced only in milligram amounts. Curium is similar in some regards to gadolinium, its rare-earth homolog, but it has a more complex crystal structure. Curium is silver in color, is chemically reactive, and is more electropositive than aluminum. CmO2, Cm2O3, CmF3, CmF4, CmCl3, CmBr3, and CmI3 have been prepared. Most compounds of trivalent curium are faintly yellow in color. 242Cm generates about three watts of thermal energy per gram. This compares to one-half watt per gram of 238Pu. This suggests use for curium as a power source. 244Cm is now offered for sale by the O.R.N.L. at $185/mg plus packing charges. 248Cm is available at a cost of $160/μg, plus packing charges, from the O.R.N.L. Curium absorbed into the body accumulates in the bones, and is therefore very toxic as its radiation destroys the red-cell forming mechanism. The maximum permissible total body burden of 244Cm (soluble) in a human being is 0.3 μCi (microcurie).
Characteristics
Curium is a synthetic (not natural) transuranic element of the actinide series. It was determinedthat curium’s major valence and oxidation state was +3, similar to other elements of thisseries. The most stable isotope of curium is curium-247, with a half-life of 1.56×10+7years.
The Uses of curium
There are no major commercial uses for curium because of the extremely small amountproduced. In the future, the most important use of curium may be to provide the power forsmall, compact thermoelectric sources of electricity, by generating heat through the nucleardecay of radioisotope curium-241. These small, efficient power sources can be used in individualhomes or remote regions to provide electricity to areas that cannot secure it from othersources. It could also be used as a source of electricity in spacecraft. However, today curium’smain use is for basic scientific laboratory research.
The Uses of curium
242Cm and 244Cm as power sources in radionuclide batteries for space and medical applications. 242Cm as radioactive heat source. 248Cm in accelerator studies to form superheavy elements.
Definition
A highly toxic radioactive silvery element of the actinoid series of metals. A transuranic element, it is not found naturally on Earth but is synthesized from plutonium. Curium-244 and curium-242 have been used in thermoelectric power generators.
Definition
curium: Symbol Cm. A radioactivemetallic transuranic element belongingto the actinoids; a.n. 96; massnumber of the most stable isotope247 (half-life 1.64 × 107 years); r.d.(calculated) 13.51; m.p. 1340±40°C.There are nine known isotopes. Theelement was first identified by GlennSeaborg (1912–99) and associates in1944 and first produced by L. B.Werner and I. Perlman in 1947 bybombarding americium–241 withneutrons.
Hazard
Curium metal and its compounds are radioactive bone-seeking poisons that attack theskeletal system of humans and animals. Care must be used in handling them.
Properties of curium
| Melting point: | 1345° |
| Boiling point: | bp (calc) 3110° |
| Density | 13.51; d 12.9 |
| form | silvery metal |
| color | silvery metal; hexagonal, hexane or
cubic |
| EPA Substance Registry System | Curium (7440-51-9) |
Safety information for curium
Computed Descriptors for curium
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8