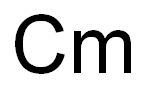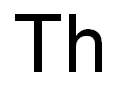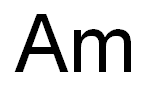protactinium
- Empirical Formula: Pa
- Molecular Weight: 231.03588

What is protactinium?
Physical properties
Protactinium is a relatively heavy, silvery-white metal that, when freshly cut, slowly oxidizesin air. All the isotopes of protactinium and its compounds are extremely radioactiveand poisonous. Proctatinium-231, the isotope with the longest half-life, is one of the scarcestand most expensive elements known. It is found in very small quantities as a decay productof uranium mixed with pitchblende, the ore of uranium. Protactinium’s odd atomic number(91Pa) supports the observation that elements having odd atomic numbers are scarcer thanthose with even atomic numbers.
Its melting point is just under 1,600°C, its boiling point is about 4,200°C, and its densityis 15.37g/cm3.
Isotopes
There are a total of 30 isotopes of protactinium. All are radioactive, and noneare stable. Their decay modes are either alpha or beta decay or electron capture. Theirhalf-lives range from 53 nanoseconds to 3.276×10+4years.
Origin of Name
A combination of the Greek word protos, meaning first, combined with the element actinium, which together means “before actinium.”
Occurrence
As mentioned, protactinium is one of the rarest elements in existence. Although protactiniumwas isolated, studied, and identified in 1934, little is known about its chemical andphysical properties since only a small amount of the metal was produced. Its major sourceis the fission by-product of uranium found in the ore pitchblende, and only about 350 milligramscan be extracted from each ton of high-grade uranium ore. Protactinium can also beproduced by the submission of samples of throrium-230 (90Th) to radiation in nuclear reactorsor particle accelerators, where one proton and one or more neutrons are added to eachthorium atom, thus changing element 90 to element 91.
History
The first
isotope of Element 91 to be discovered was 234Pa, also known
as UX2, a short-lived member of the naturally occurring 238U
decay series. It was identified by K. Fajans and O. H. Gohring
in 1913 and they named the new element brevium. When the
longer-lived isotope 231Pa was identified by Hahn and Meitner consistent with the characteristics of the most abundant isotope.
Soddy, Cranson, and Fleck were also active in this work.
The name protoactinium was shortened to protactinium in
1949. In 1927, Grosse prepared 2 mg of a white powder, which
was shown to be Pa2O5. Later, in 1934, from 0.1 g of pure Pa2O5
he isolated the element by two methods, one of which was by
converting the oxide to an iodide and “cracking” it in a high
vacuum by an electrically heated filament.
Protactinium has a bright metallic luster that it retains for
some time in air. The element occurs in pitchblende to the
extent of about 1 part 231Pa to 10 million of ore. Ores from
Congo-Kinshasa have about 3 ppm. Protactinium has twenty-
eight isotopes and isomers, the most common of which
is 231Pr with a half-life of 32,500 years. A number of protactinium
compounds are known, some of which are colored.
The element is superconductive below 1.4 K. The element is a
dangerous toxic material and requires precautions similar to
those used when handling plutonium. In 1959 and 1961, it was
announced that the Great Britain Atomic Energy Authority
extracted by a 12-stage process 125 g of 99.9% protactinium,
the world’s only stock of the metal for many years to come.
The extraction was made from 60 tons of waste material at a
cost of about $500,000. Protactinium is one of the rarest and
most expensive naturally occurring elements.
Characteristics
Because the proportion of protactinium to its ores is of the magnitude of one part in tenmillion, it takes many truckloads of ore to extract a small quantity of the metal. About 30years ago, approximately 125 grams of protactinium was extracted from over 60 tons of ore at a cost of over $500,000. These 125 grams represent the total amount of protactenium thatexists in the entire world today.
The Uses of protactinium
Protactinium is very rare, and not enough of it is available for commercial use. It is usedonly in laboratory research.
Definition
A radioactive element of atomic number 91, a member of the actinide series, aw 231.0359, valences = 4, 5; 13 unstable isotopes, two of which occur naturally. Protactinium is a con- stituent of all uranium ores, 340 mg being extracted from 1 ton. Protactin
Definition
A toxic radioactive element of the actinoid series of metals. It occurs in minute quantities in uranium ores as a radioactive decay product of actinium. Symbol: Pa; m.p. 1840°C; b.p. 4000°C (approx.); r.d. 15.4 (calc.); p.n. 91; most stable isotope 231Pa (half-life 32 500 years).
Definition
protactinium: Symbol Pa. A radioactivemetallic element belonging tothe actinoids; a.n. 91; r.a.m.231.036; r.d. 15.37 (calculated); m.p.<1600°C (estimated). The most stableisotope, protactinium–231, has ahalf-life of 3.43 × 104 years; at leastten other radioisotopes are known.Protactinium–231 occurs in all uraniumores as it is derived from uranium–235. Protactinium has nopractical applications; it was discovered by Lise Meitner and Otto Hahnin 1917.
Hazard
Highly toxic, radioactive material.
Hazard
All the isotopes of protactinium are highly radioactive poisons and therefore very dangerous.
Safety information for protactinium
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8