Californium
- CAS NO.:7440-71-3
- Empirical Formula: Cf
- Molecular Weight: 0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:43

What is Californium?
Description
Californium does not occur in nature. The element was synthesized in 1949 at the Lawrence Berkeley Laboratory in Berkeley, California by Thompson, Ghiorso and Seaborg (Thompson, S.G., Ghiorso, A. and G. T. Seaborg. 1950. Phys. Rev., 77, 838). It has 12 isotopes. It is the fifth man-made transuranium element. Presently, the element has no commercial application.
Chemical properties
α-form: hexagonal, a=0.339 nm, c=1.101nm; β: fcc, a=0.494nm; γ: fcc, a=0.575 nm; ionic radius of Cf+++ is 0.0934nm, of Cf+++ is 0.0851 nm; discovered in 1950; 252Cf is an intense neutron source, 1g emits 2.4×10+12 neutrons per sec; has application in neutron activation analysis and field use in mineral prospecting and oil-well logging, potential use in medical applications [KIR78]
Physical properties
Californium is a synthetic radioactive transuranic element of the actinide series. The puremetal form is not found in nature and has not been artificially produced in particle accelerators.However, a few compounds consisting of californium and nonmetals have been formedby nuclear reactions. The most important isotope of californium is Cf-252, which fissionsspontaneously while emitting free neutrons. This makes it of some use as a portable neutronsource since there are few elements that produce neutrons all by themselves. Most transuranicelements must be placed in a nuclear reactor, must go through a series of decay processes, ormust be mixed with other elements in order to give off neutrons. Cf-252 has a half-life of 2.65years, and just one microgram (0.000001 grams) of the element produces over 170 millionneutrons per minute.
Californium’s melting point is ~900°C, its boiling point is unknown, and its density is alsounknown.
Isotopes
There are a total of 21 isotopes of californium. None are found in nature and allare artificially produced and radioactive. Their half-lives range from 45 nanoseconds forcalifornium-246 to 898 years for californium-251, which is its most stable isotope andwhich decays into curium-247 either though spontaneous fission or by alpha decay.
Origin of Name
Named for both the state of California and the University of California.
Occurrence
Neither californium nor its compounds are found in nature. All of its isotopes are producedartificially in extremely small amounts, and all of them are extremely radioactive. All of itsisotopes are produced by the transmutation from other elements such as berkelium and americium.Following is the nuclear reaction that transmutates californium-250 into californium-252: 250Cf + (neutron and λ gamma rays) → 251Cf + (neutron and λ gamma rays) → 252Cf.
History
Californium, the sixth transuranium element to be discovered, was produced by Thompson, Street, Ghioirso, and Seaborg in 1950 by bombarding microgram quantities of 242Cm with 35 MeV helium ions in the Berkeley 60-inch cyclotron. Californium (III) is the only ion stable in aqueous solutions, all attempts to reduce or oxidize californium (III) having failed. The isotope 249Cf results from the beta decay of 249Bk while the heavier isotopes are produced by intense neutron irradiation by the reactions: 249 250 Bk(n,γ)→250 Bk?β?→ Cf and 249Cf(n,γ)→250 Cf followed by 250Cf(n,γ)→251 Cf(n,γ)→252 Cf The existence of the isotopes 249Cf, 250Cf, 251Cf, and 252Cf makes it feasible to isolate californium in weighable amounts so that its properties can be investigated with macroscopic quantities. Californium-252 is a very strong neutron emitter. One microgram releases 170 million neutrons per minute, which presents biological hazards. Proper safeguards should be used in handling californium. Twenty isotopes of californium are now recognized. 249Cf and 252Cf have half-lives of 351 years and 900 years, respectively. In 1960 a few tenths of a microgram of californium trichloride, CfCl3, californium oxychloride, CfOCl, and californium oxide, Cf2O3, were first prepared. Reduction of californium to its metallic state has not yet been accomplished. Because californium is a very efficient source of neutrons, many new uses are expected for it. It has already found use in neutron moisture gages and in well-logging (the determination of water and oil-bearing layers). It is also being used as a portable neutron source for discovery of metals such as gold or silver by on-the-spot activation analysis. 252Cf is now being offered for sale by the Oak Ridge National Laboratory (O.R.N.L.) at a cost of $60/μg and 249Cf at a cost of $185/μg plus packing charges. It has been suggested that californium may be produced in certain stellar explosions, called supernovae, for the radioactive decay of 254Cf (55-day half-life) agrees with the characteristics of the light curves of such explosions observed through telescopes. This suggestion, however, is questioned. Californium is expected to have chemical properties similar to dysprosium.
Characteristics
Californium is a transuranic element of the actinide series that is homologous with dysprosium(66Dy), just above it in the rare-earth lanthanide series. Cf-245 was the first isotopeof californium that was artificially produced. It has a half-life of just 44 minutes. Isotopes ofcalifornium are made by subjecting berkelium to high-energy neutrons within nuclear reactors,as follows: 249Bk + (neutrons and λ gamma rays) → 250Bk → 250Cf + β- (beta particleemission).
The Uses of Californium
Californium’s uses are limited, which is why the U.S. Nuclear Regulatory Commission,which controls the output and use of radioisotopes, has made californium-252 available forcommercial use at the cost of only $10 per millionth of a gram. This small quantity is adequatefor many sources of free neutrons to be used commercially. For example, free neutrons can be used in devices to measure moisture in products, including the Earth’s crust, to find wateror supplies of underground oil. Cf-252’s ability to produce neutrons has also found uses inmedicine. Cf-252’s natural spontaneous fission makes it an ideal and accurate counter forelectronic systems.
The Uses of Californium
252Cf as neutron source; startup source for nuclear reactors; in nuclear reactor fuel rod scanners; for neutron radiography of weapons components.
Definition
A silvery radioactive transuranic element of the actinoid series of metals, not found naturally on Earth. Several radioisotopes have been synthesized, including californium- 252, which is used as an intense source of neutrons in certain types of portable detector and in the treatment of cancer.
Definition
californium: Symbol Cf. A radioactivemetallic transuranic elementbelonging to the actinoids; a.n. 98;mass number of the most stable isotope251 (half-life about 700 years).Nine isotopes are known; californium–252 is an intense neutronsource, which makes it useful in neutronactivation analysis and potentiallyuseful as a radiation source inmedicine. The element was first producedby Glenn Seaborg (1912–99)and associates in 1950.
Preparation
All isotopes of the element are synthesized in the nuclear reactor. The first isotope synthesized had the mass 241, produced by irradiation of milligram quantities of americium-241 with alpha particles of 35 MeV in a cyclotron:
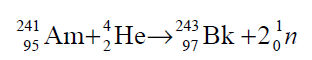
The product was separated by ion exchange
While the lighter isotopes are prepared by alpha particle bombardment, the heavier ones by neutron irradiation of large quantities of americium, curium or plutonium:
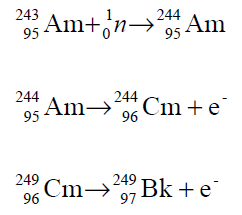
Only a small fraction of Bk-249 is obtained by the above reaction because neutrons also induce fission. Alternatively, uranium-238 may be converted to Bk-249 by very short but intense neutron bombardment followed by five successive beta decays.
Production Methods
Isotopes of californium may be produced in a cyclotron by neutron irradia tion or charged particle bombardment. Lighter isotopes of californium may be produced by bombardment of curium-242 or curium-244 with alpha particles having 35.5 MeV energy:
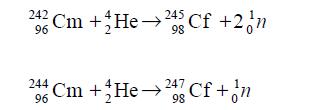
The above method was used for producing californium-245 during its first ever synthesis. Heavier isotopes of californium may be obtained by intense neutron irradiation:
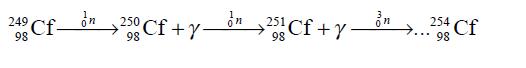
This, in turn is produced by successive slow neutron irradiation of curi um-244: Californium-254 may be produced by thermonuclear explosion resulting in the reaction of uranium-238 with intense neutron flux followed by a sequence of β- decays (Cunningham, B. B. 1968. In Encyclopedia of Chemical Elements, ed. Clifford A. Hampel, New York: Reinhold Book Co.)
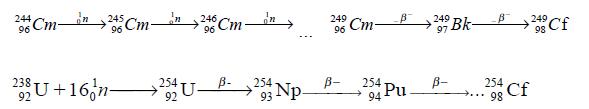
Californium is separated from other elements by fractionation and precipita tion, and further purified by solvent extraction or ion exchange.
Hazard
Californium’s greatest danger is as a biological bone-seeking radioactive element, whichcan be both a radiation hazard and a useful treatment for bone cancer. If mishandled, all ofcalifornium’s isotopes and compounds can be a potential radiation poison.
Properties of Californium
| Melting point: | 900±30° (Haire, Baybarz) |
| Density | 15.10; d 8.74 (Katz et al., loc. cit. vol. 2, p. 1150) |
| form | hexagonal or cubic metal |
| color | hexagonal, hexane or cubic metal |
Safety information for Californium
Computed Descriptors for Californium
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8