Alprenolol
- CAS NO.:13655-52-2
- Empirical Formula: C15H23NO2
- Molecular Weight: 249.35
- MDL number: MFCD00242735
- EINECS: 237-140-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
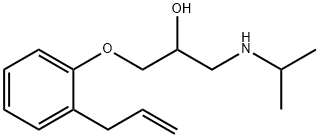
What is Alprenolol?
Description
Occupational cases of contact dermatitis caused by exposure to alprenolol have been reported within the pharmaceutical industry.
Originator
Aptol,Globopharm,Switz.
The Uses of Alprenolol
Antiadrenergic (β-receptor).
Definition
ChEBI: A secondary alcohol that is propan-2-ol substituted by a 2-allylphenoxy group at position 1 and an isopropylamino group at position 3. It is a beta-adrenergic antagonist used as a antihypertensive, anti-arrhythmia and a sympatholytic agent.
Manufacturing Process
A solution of 24.6 g of o-allyl-epoxypropoxybenzene dissolved in 250 ml of
absolute ethanol saturated with ammonia was placed in an autoclave and
heated on a steam-bath for 2 hours. The alcohol was then removed by
distillation and the residue was redissolved in a mixture of methanol and
ethylacetate. Hydrogen chloride gas was introduced into the solution. The
hydrochloride salt was then precipitated by the addition of ether to yield 11.4
g of product. Five grams of the amine-hydrochloride thus formed were
dissolved in 50 ml of methanol and 9 ml of acetone. The resulting solution
was cooled to about 0°C. At this temperature 5 g of sodium borohydride were
added over a period of 1 hour. Another 2.2 ml of acetone and 0.8 g of sodium
borohydride were added and the solution was kept at room temperature for 1
hour, after which 150 ml of water were added to the solution. The solution
was then extracted with three 100-ml portions of ether which were combined,
dried over potassium carbonate, and evaporated. The free base was then
recrystallized from petrol ether (boiling range 40-60°C) to yield 2.7 g of
material having a melting point of 57°C.
The corresponding hydrochloride was prepared by dissolving 2 g of the
product, prepared above, in 20 ml of acetone, and adding to the resulting
solution acetone saturated with hydrogen chloride until the pH was reduced to
about 3. The precipitated hydrochloride salt was then recrystallized from
acetone.
Therapeutic Function
Beta-adrenergic blocker
Contact allergens
Occupational cases of contact dermatitis due to this betablocker were reported in the pharmaceutical industry
Properties of Alprenolol
| Melting point: | 57.5°C |
| Boiling point: | 392.45°C (rough estimate) |
| Density | 1.0065 (rough estimate) |
| refractive index | 1.5250 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMF: 20 mg/ml; DMSO: 15 mg/ml; Ethanol: 5 mg/ml; PBS (pH 7.2): 1 mg/ml |
| form | A crystalline solid |
| pka | pKa 9.63 (Uncertain) |
| color | White to off-white |
| Water Solubility | 366.9mg/L(22.5 ºC) |
| NIST Chemistry Reference | 2-Propanol, 1-[(1-methylethyl)amino]-3-[2-(2-propenyl)phenoxy]-(13655-52-2) |
Safety information for Alprenolol
Computed Descriptors for Alprenolol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
