Esmolol
- CAS NO.:81147-92-4
- Empirical Formula: C16H25NO4
- Molecular Weight: 295.37
- MDL number: MFCD00864566
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-21 10:59:17
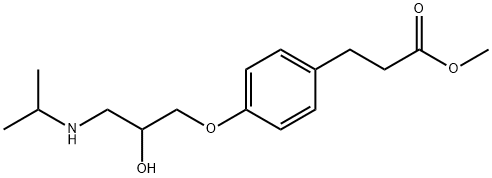
What is Esmolol?
Absorption
Rapidly absorbed, steady-state blood levels for dosages from 50-300 μg/kg/min (0.05-0.3 mg/kg/mm) are obtained within five minutes.
Toxicity
Symptoms of overdose include cardiac arrest, bradycardia, hypotension, electromechanical dissociation and loss of consciousness.
Originator
Esmolol,AroKor Holdings
The Uses of Esmolol
Esmolol is a cardioselective beta1 receptor blocking agent.
The Uses of Esmolol
Agricultural chemical.
Indications
For the rapid control of ventricular rate in patients with atrial fibrillation or atrial flutter in perioperative, postoperative, or other emergent circumstances where short term control of ventricular rate with a short-acting agent is desirable. Also used in noncompensatory sinus tachycardia where the rapid heart rate requires specific intervention.
Background
Esmolol, commonly marketed under the trade name Brevibloc, is a cardioselective beta-1 receptor blocker. It has a rapid onset but short duration of action without causing significant intrinsic sympathomimetic or membrane stabilizing activities at recommended therapeutic doses. It works by blocking beta-adrenergic receptors in the heart, which leads to decreased force and rate of heart contractions. Esmolol prevents the action of two naturally occurring substances: epinephrine and norepinephrine.
The FDA withdrew its approval for the use of all parenteral dosage form drug products containing esmolol hydrochloride that supply 250 milligrams/milliliter of concentrated esmolol per 10-milliliter ampule. Other esmolol formulations are still available for use.
What are the applications of Application
Esmolol is a cardioselective beta1 receptor blocking agent
Definition
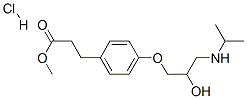
ChEBI: Methyl 3-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}propanoate is a methyl ester that is methyl 3-(4-hydroxyphenyl)propanoate in which the hydrogen attached to the phenolic hydroxy group is substituted by a 2-hydroxy-3-(isopropylamino)propyl group. It is an aromatic ether, a member of ethanolamines, a methyl ester, a secondary alcohol and a secondary amino compound. It is functionally related to a 3-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}propanoic acid.
Manufacturing Process
A solution of 17 g (0.1 mole) of 3-(4-hydroxyphenyl)propionic acid in 500 mL
methanol and 2 mL concentrated sulfuric acid were placed in a Soxhlet
extractor charged with 3A molecular sieves. The solution was refluxed for 72
hours and the sieve were exchanged at 24 hour intervals. The reaction
medium was then evaporated to an oil which was dissolved in 100 mL toluene
and extracted with 100 mL water (3 times). The toluene phase was dried over
magnesium sulfate, treated with activated charcoal and evaporated to provide
15 g (80%) of a clear oil. The NMR spectrum was consistent with the methyl
3-(4-hydroxyphenyl)propionate.
The oil described above was utilized directly in the condensation reaction with
the epichlorohydrin. A mixture of 0.1 mole of methyl 3-(4-
hydroxyphenyl)propionate, 0.2 mole potassium carbonate and 0.4 mole
epichlorohydrin in 250 mL acetone was heated to reflux for 24 hours. The
reaction medium was then filtered and evaporated. The residue was taken up
in 100 mL toluene and washed with 100 mL 1.0 N NaOH and 100 mL water (2
times). The toluene phase was then dried over magnesium sulfate and
evaporated to provide the crude product as an oil. Purification was effected by
vacuum distillation (156°C/0.4 mm) and provided methyl 3-[4-(2,3-
epoxypropoxy)phenyl]propionate. The NMR and IR spectra and elemental
analysis data were consistent with the assigned structure.
A mixture of 50 g (0.21 mole) of methyl 3-[4-(2,3-
epoxypropoxy)phenyl]propionate and 100 mL of isopropylamine in 100 mLmethanol was heated to reflux for 4 hours. The reaction medium was then
evaporated and the resulting oil taken up in methanol and treated with
ethereal HCl and provided crystals which were recrystallized in similar fashion
to provide 28 g (47%) of white crystals: melting point 85-86°C. The NMR and
IR spectra and the elemental analysis data were consistent with the structure
of methyl 4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)benzenepropanoate.
In practice it is usually used as hydrochloride.
Therapeutic Function
Beta-adrenergic blocker
Hazard
A reproductive hazard.
Clinical Use
Esmolol (Brevibloc) is a short-acting intravenously administered
β1-selective adrenoceptor blocking agent. It
does not possess membrane-stabilizing activity or sympathomimetic
activity.
Esmolol is used in the treatment of supraventricular
tachyarrhythmias for rapid control of ventricular rate
and reduction of myocardial oxygen consumption.
Discontinuation of administration is followed by a rapid
reversal of its pharmacological effects because of esmolol’s
rapid hydrolysis by plasma esterases.
Side Effects
The most frequently reported adverse effects are hypotension, nausea, dizziness, headache, and dyspnea.As with many β-blocking drugs, esmolol is contraindicated in patients with overt heart failure and those in cardiogenic shock.
Metabolism
Esmolol undergoes rapid hydrolysis of ester linkage which is catalyzed by esterases found in the cytosol of red blood cells (RBCs). The plasma cholinersterases or RBC membrane acetylcholinesterases are not involved in this metabolic reaction. Metabolism of the drug occurs mainly in RBCs to form a free acid metabolite (with 1/1500 the activity of esmolol) and methanol.
Metabolism
The -blocker esmolol (Brevibloc) is unusual in that it is very rapidly metabolized; its plasma half-life is only 9 minutes. It is subject to hydrolysis by cytosolic esterases in red blood cells to yield methanol and an acid metabolite, the latter having an elimination half-life of about 4 hours. Only 2% of the administered esmolol is excreted unchanged. Because of its rapid onset and short duration of action, esmolol is used by the intravenous route for the control of ventricular arrhythmias in emergencies.
Properties of Esmolol
| Melting point: | 48-50 °C |
| Boiling point: | 430.2±40.0 °C(Predicted) |
| Density | 1.026 |
| storage temp. | Store at -20°C |
| solubility | DMSO: 59 mg/mL (199.75 mM);Ethanol: 59 mg/mL (199.75 mM) |
| pka | 13.88±0.20(Predicted) |
| Water Solubility | Slightly soluble |
| Merck | 14,3700 |
| CAS DataBase Reference | 81147-92-4(CAS DataBase Reference) |
Safety information for Esmolol
Computed Descriptors for Esmolol
New Products
ALUMINIUM IODIDE 100 GM BUFFER CAPSULE PH 7.0 - 10 CAP BUFFER SOLUTION PH 9.5 (BORATE) EZEE BLUE GEL STAINER BORAX CARMINE (GRENACHERS ALCOHOLIC) POTASSIUM IODATE - IODIDE SOLN 0.1 N Dabigatran Acyl-O3-D-Glucuronide Trifluoroacetic Acid Salt Isofolic Acid Dabigatran 2-O-acylglucuronide metabolite Dabigatran Acyl-?-D- glucuronide Trifluroacetic Acid Erythromycin EP Impurity A Desloratidine Related Compound ARelated products of tetrahydrofuran




You may like
-
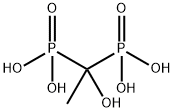
 Esmolol 95% CAS 81147-92-4View Details
Esmolol 95% CAS 81147-92-4View Details
81147-92-4 -
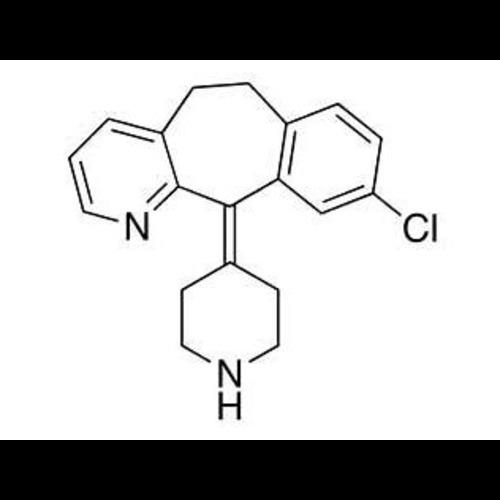 Dechloro DesloratadineView Details
Dechloro DesloratadineView Details -
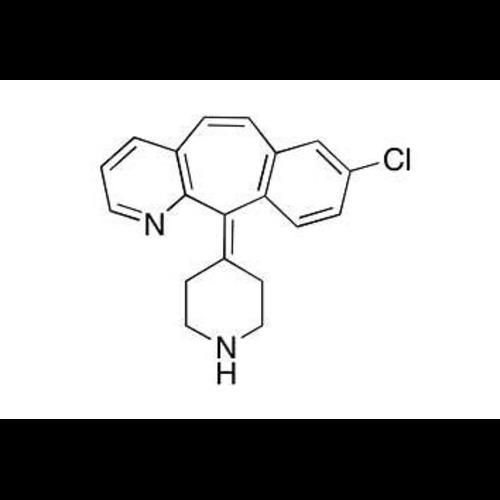 Dehydro DesloratadineView Details
Dehydro DesloratadineView Details -
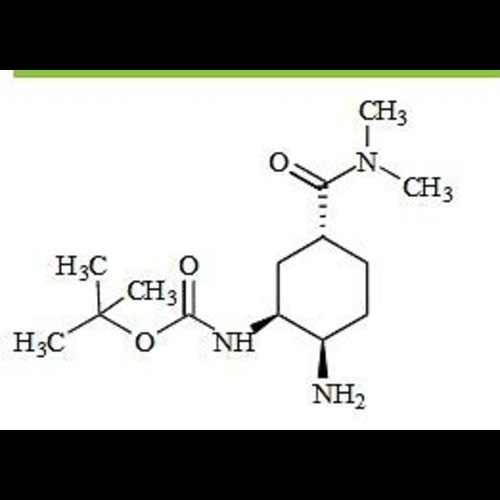 Edoxaban Impurity 57View Details
Edoxaban Impurity 57View Details
2089454-69-1 -
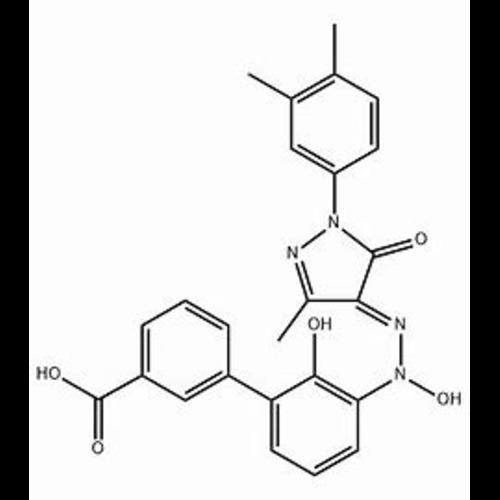 Eltrombopag N-Oxide ImpurityView Details
Eltrombopag N-Oxide ImpurityView Details
2734533-17-4 -
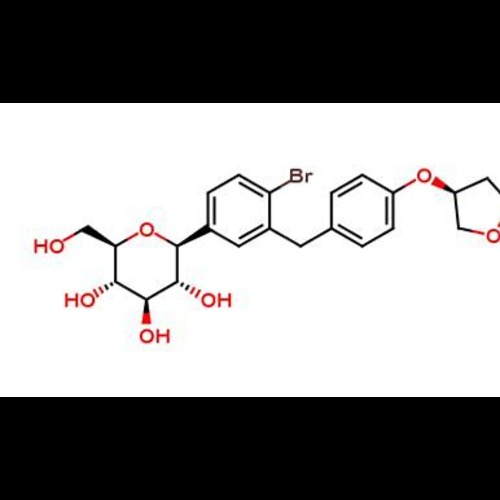 Empagliflozin Bromo ImpurityView Details
Empagliflozin Bromo ImpurityView Details -
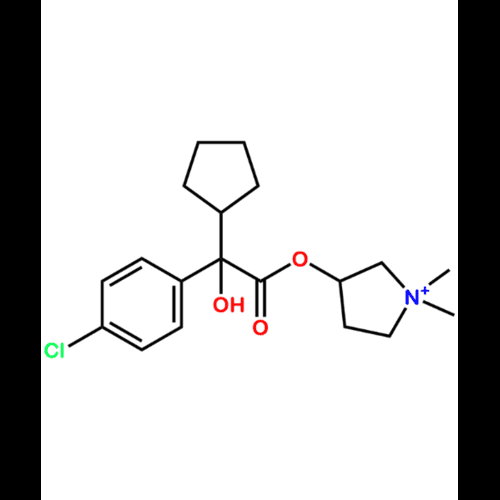 Glycopyrronium Bromide EP Impurity IView Details
Glycopyrronium Bromide EP Impurity IView Details
1404617-94-2 -
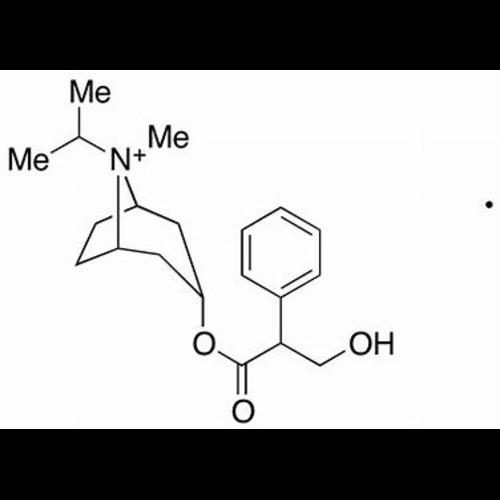 Ipratropium EP Impurity BView Details
Ipratropium EP Impurity BView Details
58073-59-9