Bisoprolol
Synonym(s):1-{4-{[2-(1-Methylethoxy)ethoxy]methyl}phenoxy}-3-[(1-methylethyl)amino]-2-propanol
- CAS NO.:66722-44-9
- Empirical Formula: C18H31NO4
- Molecular Weight: 325.44
- MDL number: MFCD00865795
- EINECS: 613-980-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
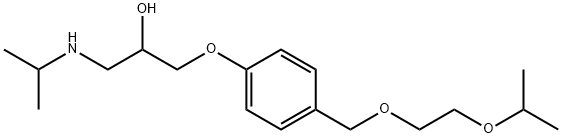
What is Bisoprolol?
Absorption
Bisoprolol is well absorbed in the gastrointestinal tract. The AUC is 642.87 g.hr/mL and bioavailability of bisoprolol is about 90% due to the minimal first pass effects. Absorption is unaffected by food intake. Peak plasma concentrations of bisoprolol are attained within 2-4 hours and steady-state concentrations are achieved within 5 days of administration. In a pharmacokinetic study, the mean peak concentration of bisoprolol was 52 micrograms/L. Cmax at steady state concentrations of bisoprolol is 64±21 ng/ml administered at 10 mg daily.
Toxicity
LD50 information
Oral LD50 of bisoprolol in the mouse was 730 mg/kg.
Overdose information
Signs of a β-blocker overdose include cardiovascular symptoms such as hypotension, congestive heart failure, and bradycardia. Other symptoms such as bronchospasm, and hypoglycemia may occur. If an overdose occurs with bisoprolol, supportive treatment should be initiated. Glucagon has been shown to be beneficial in bradycardia and hypotension associated with beta-blocker overdosage. Hypoglycemia may be managed by administering IV glucose. Monitor the patient and administer atropine in cases of bradycardia, pressors and fluids in the case of hypotension, and conventional heart failure therapy if heart failure occurs. If heart block occurs, the patient must be closely monitored and isoproterenol infusion or transvenous cardiac pacemaker insertion should take place. For the management of overdose-related bronchospasm, administer bronchodilators with or without IV aminophylline. Limited research suggests that bisoprolol fumarate is not removed adequately by hemodialysis sessions.
Chemical properties
Yellow Oil
The Uses of Bisoprolol
Cardensiel is an intermediate in the synthesis of 3,3''-(Isopropylazanediyl)bis(1-(4-((2-isopropoxyethoxy)methyl)phenoxy)propan-2-ol) (I824005), which is an impurtiy of Bisoprolol (B510500), a selective β-adrenergic blocker. Used as an antihypertensive.
The Uses of Bisoprolol
A selective a-adrenergic blocker. Used as an antihypertensive
Indications
Bisoprolol is indicated for the treatment of mild to moderate hypertension. It may be used off-label to treat heart failure, atrial fibrillation, and angina pectoris.
Background
Bisoprolol is a cardioselective β1-adrenergic blocking agent used to treat high blood pressure. It is considered a potent drug with a long-half life that can be used once daily to reduce the need for multiple doses of antihypertensive drugs. Bisoprolol is generally well tolerated, likely due to its β1-adrenergic receptor selectivity and is a useful alternative to non-selective β-blocker drugs in the treatment of hypertension such as Carvedilol and Labetalol. It may be used alone or in combination with other drugs to manage hypertension and can be useful in patients with chronic obstructive pulmonary disease (COPD) due to its receptor selectivity.
What are the applications of Application
Bisoprolol-d5 is A selective β-adrenergic blocker
What are the applications of Application
Bisoprolol is an amino phenoxy compound for proteomics research
Definition
ChEBI: Bisoprolol is a secondary alcohol and a secondary amine. It has a role as an antihypertensive agent, a beta-adrenergic antagonist, an anti-arrhythmia drug and a sympatholytic agent.
Pharmacokinetics
Bisoprolol decreases heart rate (chronotropy), decreases contractility (inotropy), and reduces blood pressure. The results of various clinical studies indicate that bisoprolol reduces cardiovascular mortality and all-cause mortality in patients with heart failure and decreased cardiac ejection fraction (EF).
Enzyme inhibitor
This oral cardiospecific β-blocker (FWfree-base = 325.45 g/mol; CAS 66722- 44-9; IUPAC: (RS)-1-{4-[(2-isopropoxyethoxy)methyl]phenoxy}-3- (isopropylamino)propan-2-ol), also named Zebeta? (oral tablets as fumarate salt), selectively targets β1-adrenergic receptors, blocking epinephrine stimulation of β1-adrenoreceptors that are concentrated in the heart muscle cells and heart conduction tissue as well as kidney juxtaglomerular cells. Bisoprolol is used to treat hypertension, coronary heart disease, arrhythmias, ischemic heart diseases, and myocardial infarction after the acute event. Given such versatility, bisoprolol is understandably included in the World Health Organization's List of Essential Medicines. Pharmacokinetics: Bioavailability > 90%; Hepatic metabolism (50%) by CYP2D6 and CYP3A4; Biological t1/2 = 10-12 hours.
Metabolism
Approximately 50% of the bisoprolol dose is eliminated by non-renal pathways. Bisoprolol is metabolized through oxidative metabolic pathways with no subsequent conjugation. Bisoprolol metabolites are polar and, therefore, really eliminated. Major metabolites found in plasma and urine are inactive. Bisoprolol is mainly metabolized by CYP3A4 (95%), whereas CYP2D6 plays a minor role. The CYP3A4-mediated metabolism of bisoprolol appears to be non-stereoselective.
Properties of Bisoprolol
| Melting point: | 100 °C |
| Boiling point: | 445.0±45.0 °C(Predicted) |
| Density | 1.033±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| form | neat |
| pka | pKa 9.57(H2O(extrap) t=25±1 Iunde?ned Aratmosphere) (Uncertain) |
| color | Yellow |
| Water Solubility | 5.5ug/L(100 ºC) |
| CAS DataBase Reference | 66722-44-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Bisoprolol(66722-44-9) |
Safety information for Bisoprolol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H362:Reproductive toxicity, effects on or via lactation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P263:Avoid contact during pregnancy/while nursing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Bisoprolol
Bisoprolol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
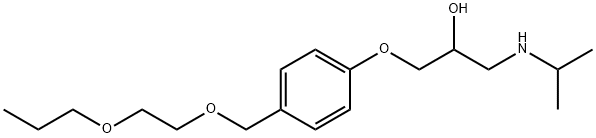
![2-Propanol, 1,1'-[methylenebis(4,1-phenyleneoxy)]bis[3-[(1-methylethyl)amino]-](https://img.chemicalbook.in/CAS/20200515/GIF/1225195-70-9.gif)



You may like
-
![66722-44-9 (RS)-1-{4-[(2-isopropoxyethoxy)methyl]phenoxy}-3-(isopropylamino)propan-2-ol 99%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/cd343a71-844b-48e7-83b6-79cc47de5812.png) 66722-44-9 (RS)-1-{4-[(2-isopropoxyethoxy)methyl]phenoxy}-3-(isopropylamino)propan-2-ol 99%View Details
66722-44-9 (RS)-1-{4-[(2-isopropoxyethoxy)methyl]phenoxy}-3-(isopropylamino)propan-2-ol 99%View Details
66722-44-9 -
 Bisoprolol 98%View Details
Bisoprolol 98%View Details
66722-44-9 -
 Bisoprolol 66722-44-9 98%View Details
Bisoprolol 66722-44-9 98%View Details
66722-44-9 -
 Bisoprolol 66722-44-9 98%View Details
Bisoprolol 66722-44-9 98%View Details
66722-44-9 -
 66722-44-9 Bisoprolol 99%View Details
66722-44-9 Bisoprolol 99%View Details
66722-44-9 -
 66722-44-9 98%View Details
66722-44-9 98%View Details
66722-44-9 -
 Bisoprolol 98% (GC) CAS 66722-44-9View Details
Bisoprolol 98% (GC) CAS 66722-44-9View Details
66722-44-9 -
 Bisoprolol CAS 66722-44-9View Details
Bisoprolol CAS 66722-44-9View Details
66722-44-9
