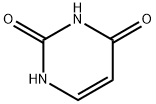
Alloxan
- CAS NO.:50-71-5
- Empirical Formula: C4H2N2O4
- Molecular Weight: 142.07
- MDL number: MFCD00006031
- EINECS: 200-062-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
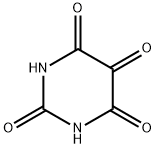
What is Alloxan?
Description
Alloxan is a thermally stable white solid with an unusual structure. Its formal name is 2,4,5,6(1H,3H)-pyrimidinetetrone, indicating that it has a pyrimidine ring structure with four carbonyl groups. It is freely soluble in water, forming a slightly acidic solution. The solid begins to decompose at 256 oC.
In 1818, Italian chemist Luigi V. Brugnatelli was the first to isolate alloxan; he synthesized it via nitric acid oxidative degradation of uric acid. Shortly after Friedrich W?hler found that urea can be made from inorganic materials in 1828, he and Justus von Liebig discovered alloxan in human excretions, showing that it also can be biosynthesized. Currently, alloxan is prepared from barbituric acid or alloxantin; the article of commerce is the monohydrate.
Alloxan is sometimes used in dye manufacture, but its main (and most notorious) use is to induce diabetes in laboratory rodents. Alloxan’s structure mimics that of glucose, which allows it to be absorbed by the pancreas. Once inside the organ, it destroys insulin-producing β-cells and produces a disease similar to type 1 diabetes in humans. Fortunately, alloxan is not taken up by the human pancreas, but it has shown liver and kidney toxicity.
Chemical properties
Alloxan is a white crystals, become pink on exposure to air; colorless aqueous solution which imparts pink color to skin. Soluble in water and alcohol.
The Uses of Alloxan
Alloxan is used in biochemical research, cosmetics, organic synthesis.
Definition
ChEBI: A member of the class of pyrimidones, the structure of which is that of perhydropyrimidine substituted at C-2, -4, -5 and -6 by oxo groups.
Safety Profile
Poison by intraperitoneal, intravenous, subcutaneous, and rectal routes. Moderately toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. Produces dlabetes in experimental animals. Decomposes in storage to release CO2. Do not store in sealed container. Explodes when heated above 170℃. When heated to decomposition it emits toxic fumes of NOx,.
Purification Methods
Crystallisation from water gives the tetrahydrate. Anhydrous crystals are obtained by crystallisation from acetone, glacial acetic acid or by sublimation in vacuo. [See below and Beilstein 24 H 500, 24 I 428, 24 II 301, 24 III/IV 2137.]
Properties of Alloxan
| Melting point: | ~245 °C (dec.) |
| Boiling point: | 259.59°C (rough estimate) |
| Density | 1.8588 (rough estimate) |
| refractive index | 1.4610 (estimate) |
| storage temp. | 2-8°C |
| pka | pK (25°) 6.63 |
| Water Solubility | 8g/L(temperature not stated) |
| CAS DataBase Reference | 50-71-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Alloxan(50-71-5) |
| EPA Substance Registry System | 2,4,5,6(1H,3H)-Pyrimidinetetrone (50-71-5) |
Safety information for Alloxan
Computed Descriptors for Alloxan
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8