Adrafinil
- CAS NO.:63547-13-7
- Empirical Formula: C15H15NO3S
- Molecular Weight: 289.35
- MDL number: MFCD00865357
- EINECS: 264-303-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:01:58
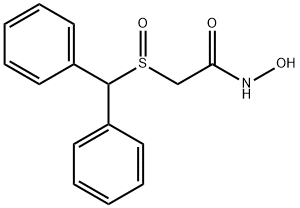
What is Adrafinil?
Description
Adrafinil is a centrally-acting, αl-adrenergic agonist useful in the management of vigillance disturbances and depression in the elderly.
Chemical properties
Light Pink Solid
Originator
Labs. L. Lafon (France)
The Uses of Adrafinil
a-Adrenergic agonist. Treatment of depression
The Uses of Adrafinil
α-Adrenergic agonist. Treatment of depression.
What are the applications of Application
Adrafinil is an α-adrenergic agonist
Definition
ChEBI: Adrafinil is a diarylmethane.
Manufacturing Process
7.6 g (0.1 mol) of thiourea and 100 ml of dematerialized water are introduced
into a 500 ml three-neck flask equipped with a magnetic stirrer, a dropping
funnel and a condenser; the mixture is heated to 50°C and 20.25 g (18 ml;
0.1 mol) of chlorodiphenylmethane are then added all at once. The solution is
left refluxing until it has become limpid, and is then cooled to 20°C, and 200
ml of 2.5 N NaOH are added dropwise. So the sodium benzhydrylthiolate is
obtained.
A solution of sodium 3-chloroacetate is added to the solution of sodium
benzhydrylthiolate at about 60°C. Thereafter the temperature is raised to the
boil, the mixture is left under reflux for about 0.5 h and is then cooled,
filtered over charcoal and acidified with concentrated HCl, and 3-
(benzhydrylthio)acetic acid are thus precipitated.
To the solution of 3-(benzhydrylthio)acetic acid in 1,2-dichloroethane,
methanol and concentrated H2SO4 have been added. The whole is heated to
the reflux temperature for about 5 h, cooled, and decanted, the aqueous
phase is discarded and the organic phase is washed with a saturated sodium
bicarbonate solution and then with water until the wash waters have a neutral
pH. After drying over MgSO4 and evaporating the solvent, the 3-
(benzhydrylthio)acetic acid methyl ester is obtained.
The 3-(benzhydrylthio)acetic acid methyl ester dissolved in methanol, is added
to a solution of hydroxylamine base [prepared by neutralising 0.15 mol (10.4
g) of hydroxylamine hydrochloride with 0.15 mol of sodium methylate]. The
whole is left at ordinary temperature (15°-25°C) for 48 h, the sodium chloride
is filtered off, the methanol is evaporated, the residue is taken up with
aqueous alkali, the solution is filtered over charcoal, the filtrate is acidified
with concentrated HCl, and the 3-(benzhydrylthio)acethylhydroxamic acid
(recrystallised from benzene) is thus obtained.
The 3-(benzhydrylthio)acethylhydroxamic acid, dissolved in anhydrous
CH3COOH, is reacted with H2O2. The mixture is left at 40°-45°C for about 1.5
h, the acetic acid is evaporated and the residue is taken up in 50 ml of ethyl
acetate; the 2-[(diphenylmethyl)sulfinyl]-N-hydroxyacetamide (CRL 40028)
crystallises (recrystallised from isopropanol).
brand name
OLMIFON
Therapeutic Function
Psychostimulant
Properties of Adrafinil
| Melting point: | 159-1600C |
| Density | 1.342±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO: 35 mg/mL |
| pka | 8.22±0.20(Predicted) |
| form | solid |
| color | white |
| CAS DataBase Reference | 63547-13-7(CAS DataBase Reference) |
Safety information for Adrafinil
Computed Descriptors for Adrafinil
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1