Dimethyl sulfoxide
Synonym(s):DMSO;Dimethyl sulfoxide;Sulfoxide;Methyl Sulfoxide;Methylsulfinylmethane
- CAS NO.:67-68-5
- Empirical Formula: C2H6OS
- Molecular Weight: 78.13
- MDL number: MFCD00002089
- EINECS: 200-664-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
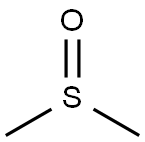
What is Dimethyl sulfoxide?
Absorption
Readily and rapidly absorbed following administration by all routes and distributed throughout the body.
Toxicity
The oral LD50 of dimethyl sulfoxide in the dog is greater than 10 gm/kg. It is improbable that this dosage level could be obtained with intravesical instillation of dimethyl sulfoxide in the patient.
Description
Dimethyl sulfoxide (DMSO) is a widely used solvent that is miscible with water and a wide range of organic solvents. It goes by several names, including methyl sulfoxide, sulfinylbismethane, and dozens of trade names. DMSO was first discovered in the late 19th century as a byproduct of the kraft process for making paper from wood pulp. About the same time, Russian chemist Alexander Zaytsev synthesized it by oxidizing dimethyl sulfide, another kraft process byproduct. Zaytsev’s synthesis is the basis for the manufacturing process still used today.
Chemical properties
Dimethyl sulfoxide occurs as a colorless, viscous liquid, or as colorless crystals that are miscible with water, alcohol, and ether. The material hasa slightly bitter taste with a sweet aftertaste, and is odorless, or has a slight odor characteristic of dimethyl sulfoxide.Dimethyl sulfoxide is extremely hygroscopic, absorbing up to 70% of its own weight in water with evolution of heat.
Occurrence
Reported found in apple, raspberry, cabbage, cucumber, onion, tomato, peppermint and spearmint oils, milk, pork liver, beer, rum, cocoa, coffee, black tea, oatmeal, soybean, beetroot, parsnip root, watercress, sweet corn, malt, cooked shrimp and oysters
The Uses of Dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is a polar aprotic solvent that can dissolve a wide range of organic compounds. It is a laboratory and industrial solvent for many gases, synthetic fibers, paint, hydrocarbons, salts, and natural products. Because it is aprotic, relatively inert, nontoxic, and stable at high temperatures, it is a frequently used solvent for chemical reactions. Its deuterated form is an ideal solvent for NMR spectroscopy.
Background
A highly polar organic liquid, that is used widely as a chemical solvent. Because of its ability to penetrate biological membranes, it is used as a vehicle for topical application of pharmaceuticals. It is also used to protect tissue during cryopreservation. Dimethyl sulfoxide shows a range of pharmacological activity including analgesia and anti-inflammation.
Indications
For the symptomatic relief of patients with interstitial cystitis.
Production Methods
Dimethyl sulfoxide is prepared by air oxidation of dimethyl sulfide in the presence of nitrogen oxides. It can also be obtained as a by product of wood pulp manufacture for the paper and allied industries.
Manufacturing Process
1) bringing hydrogen sulfide in contact with methanol to obtain a mixture containing dimethyl sulfide, and separating the dimethyl sulfide from the mixture; 2) in the presence or absence of a solvent, bringing the dimethyl sulfide obtained in step 1) in contact with at least one oxidant and a catalyst to obtain a mixture containing the dimethylsulfoxide, the catalyst containing at least one titanium silicon molecular sieve.
Air & Water Reactions
Denser than water and miscible in water.
Flammability and Explosibility
Combustible when exposed to heat or flame (NFPA rating = 1). Carbon dioxide or dry chemical extinguishers should be used to fight DMSO fires.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Pharmaceutical Applications
Dimethyl sulfoxide enhances the topical penetration of drug sowing to its ability to displace bound water from the stratumcorneum;this is accompanied by the extraction of lipids and configurational changes of proteins.
Dimethyl sulfoxide is now incorporated into a number of regulated products for healthcare and drug delivery applications, including stabilizing product formulations, sustained-release applications, and for the delivery of medical polymers.
The use of dimethyl sulfoxide to improve transdermal delivery has been reported for diclofenac, ciclosporin, timolol, and a wide range of other drugs. Dimethyl sulfoxide has also been used in the formulation of an injection containing allopurinol. It has also been investigated for use in an experimental parenteral preparation for the treatment of liver tumors.
Industrial uses
Dimethyl sulfoxide, a product of an oxidation reaction on dimethyl sulfide, contains a very polar sulfoxide functional group. This highly polar functional group enables DMSO to form complexes with many metal ions, to act as a reaction medium for synthetic reactions, and to dissolve a large number of organic resins and polymers.
Dimethyl sulfoxide is a favored solvent for displacement reactions in synthetic chemistry. The rates of reaction in DMSO are many times faster than in an alcohol or aqueous medium. Dimethyl sulfoxide is the solvent of choice in reactions where proton (hydrogen atom) removal is the rate determining step. Reactions of this type include olefin isomerizations and reactions where an elimination process produces an olefin. Another application that uses DMSO is its use as an extraction solvent to separate olefins from saturated paraffins.
Biological Activity
Solvent with wide ranging applications in biological research.
Biochem/physiol Actions
Dimethyl sulfoxide (DMSO) is a low molecular weight, less hydrophilic and a freely permeable cryoprotectant. It is a polar aprotic solvent used in polymerase chain reactions (PCR) and as a cryoprotectant and vitrification agent for the preservation of cells, tissues and organs.
Pharmacokinetics
Dimethyl Sulfoxide may have anti-inflammatory, antioxidant and analgesic activities. Dimethyl Sulfoxide also readily penetrates cellular membranes. The membrane-penetrating ability of dimethyl sulfoxide may enhance diffusion of other substances through the skin. For this reason, mixtures of idoxuridine and dimethyl sulfoxide have been used for topical treatment of herpes zoster in the United Kingdom.
Safety Profile
Slightly toxic by ingestion. Moderately toxic by intravenous and intraperitoneal routes. Human systemic effects by intravenous route: nausea or vomiting and jaundice. Experimental teratogenic and reproductive effects. A skin and eye irritant. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. Can cause an anaphylactic reaction. It freely penetrates the skin and may carry dissolved chemicals with it into the body.
Safety
Dimethyl sulfoxide has low systemic toxicity but causes local toxic effects. It is readily absorbed after injection or after oral or percutaneous administration and is widely distributed throughout the body. Dimethyl sulfoxide acts as a primary irritant on skin, causing redness, burning, itching, and scaling; it also causes urticaria. Systemic symptoms include nausea, vomiting, chills, cramps, andlethargy;dimethyl sulfoxidecanalsocauseincreases in intraocular pressure.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit
Environmental Fate
Most physiological properties of DMSO appear to be related to its penetration properties, its potential to inhibit or stimulate enzymes and to act as a free radical scavenger, and its ability to cause histamine release from mast cells. These properties are largely based on DMSO’s chemical characteristics, including its hydrogen bonding behavior, water affinity, ability to interchange with water in membranes, and ability to react with organic molecules.
Metabolism
Dimethyl sulfoxide is metabolized in man by oxidation to dimethyl sulfone or by reduction in dimethyl sulfide. Dimethyl sulfoxide and dimethyl sulfone are excreted in the urine and feces.
Shipping
DMSO is not specifically cited in DOT’sPerformance-Oriented Packaging Standards.
Incompatibilities
DMSO reacts violently with strong oxidizers, many acyl halides, boron hydrides, and alkali metals. DMSO can form explosive mixtures with metal salts of oxoacids (sodium perchlorate, iron(III) nitrate).
Properties of Dimethyl sulfoxide
| Melting point: | 18.4 °C |
| Boiling point: | 189 °C(lit.) |
| Density | 1.100 g/mL at 20 °C |
| vapor density | 2.7 (vs air) |
| vapor pressure | 0.42 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 3875 | METHYLSULFINYLMETHANE |
| Flash point: | 192 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: miscible (completely) |
| form | liquid (temperature dependent) |
| appearance | colorless liquid |
| pka | 35(at 25℃) |
| color | clear colorless |
| Relative polarity | 0.444 |
| Odor | Mild garlic odor |
| explosive limit | 1.8-63.0%(V) |
| Water Solubility | Soluble in water, methanol, acetone, ether, benzene, chloroform. |
| FreezingPoint | 18.4℃ |
| Sensitive | Hygroscopic |
| λmax | λ: 285 nm Amax: ≤0.20 λ: 295 nm Amax: ≤0.20 |
| JECFA Number | 507 |
| Merck | 14,3259 |
| BRN | 506008 |
| Dielectric constant | 41.9(55℃) |
| Stability: | Stable. Incompatible with a very wide range of materials, including acid chlorides, strong acids, strong oxidizing agents, strong reducing agents, phosphorus halides, moisture, copper wool + trichloroacetic acid. Reacts violently with a number of materials - consult a full data sheet before use. Hygroscopic. |
| CAS DataBase Reference | 67-68-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Dimethyl sulfoxide(67-68-5) |
| EPA Substance Registry System | Dimethyl sulfoxide (67-68-5) |
Safety information for Dimethyl sulfoxide
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H227:Flammable liquids H320:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P314:Get medical advice/attention if you feel unwell. P403+P235:Store in a well-ventilated place. Keep cool. P501:Dispose of contents/container to..… |
Computed Descriptors for Dimethyl sulfoxide
| InChIKey | IAZDPXIOMUYVGZ-UHFFFAOYSA-N |
Dimethyl sulfoxide manufacturer
JSK Chemicals
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 DMSO (Dimethyl sulfoxide) 98%View Details
DMSO (Dimethyl sulfoxide) 98%View Details -
 DMSO 99%View Details
DMSO 99%View Details -
 Dimethyl sulfoxide CAS 67-68-5View Details
Dimethyl sulfoxide CAS 67-68-5View Details
67-68-5 -
 Dimethyl sulfoxide, HPLC Grade 99%View Details
Dimethyl sulfoxide, HPLC Grade 99%View Details -
 Dimethyl sulfoxide, 99% 99%View Details
Dimethyl sulfoxide, 99% 99%View Details -
 Dimethyl Sulfoxide CASView Details
Dimethyl Sulfoxide CASView Details -
 Dimethyl Sulfoxide CASView Details
Dimethyl Sulfoxide CASView Details -
 Dimethyl sulfoxide 99% CASView Details
Dimethyl sulfoxide 99% CASView Details
