Actidione
Synonym(s):CHX;Actidione;Cycloheximide - CAS 66-81-9 - Calbiochem;Cycloheximide, High Purity - CAS 66-81-9 - Calbiochem;InSolution Cycloheximide - CAS 66-81-9 - Calbiochem
- CAS NO.:66-81-9
- Empirical Formula: C15H23NO4
- Molecular Weight: 281.35
- MDL number: MFCD06202064
- EINECS: 200-636-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
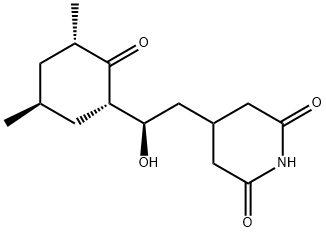
What is Actidione?
Description
Cycloheximide is a glutarimide-type antibiotic produced by Streptomyces griseus. Colorless crystals, C15H23NO4 (281.4), melting point: 115.5–117 ?C, weak acidic substance (pKa 11.2), Soluble in chloroform, isopropanol and methanol; water > 21 g/L (2 ?C). Stable in pH 3–5, but rapidly destroyed in alkaline solutions.
Description
Cycloheximide is a glutarimide antibiotic produced by S. griseus that inhibits protein synthesis in eukaryotes (IC50 = 5-
Chemical properties
Off-white to light tan powder
Chemical properties
Cycloheximide is a colorless crystalline sub- stance.
The Uses of Actidione
immunomodulator
The Uses of Actidione
Cycloheximide is an antibiotic substance isolated from the beers of streptomycin-producing strains of Streptomyces griseus. Cycloheximide is used as fungicide.
The Uses of Actidione
Cycloheximide is the most recognised member of the glutarimide microbial metabolites. Cycloheximide was isolated from Streptomyces griseus in the late 1940s as a potent and broad spectrum antifungal. Cycloheximide inhibits protein synthesis by interfering with translocation. Cycloheximide is an established bioprobe and widely-used antifungal reagent in research with over 25,000 literature citations.
The Uses of Actidione
potent and selective 5-HT uptake inhibitor
The Uses of Actidione
immunosuppressant
What are the applications of Application
Cycloheximide is a highly effective antibiotic. It inhibits the synthesis of proteins and macromolecules.
Definition
ChEBI: Cycloheximide is a dicarboximide that is 4-(2-hydroxyethyl)piperidine-2,6-dione in which one of the hydrogens attached to the carbon bearing the hydroxy group is replaced by a 3,5-dimethyl-2-oxocyclohexyl group. It is an antibiotic produced by the bacterium Streptomyces griseus. It has a role as a bacterial metabolite, a protein synthesis inhibitor, a neuroprotective agent, an anticoronaviral agent and a ferroptosis inhibitor. It is a member of piperidones, a piperidine antibiotic, an antibiotic fungicide, a dicarboximide, a secondary alcohol and a cyclic ketone. It is functionally related to a piperidine-2,6-dione.
General Description
Colorless crystals. Used as a fungicide and as a anticancer drug.
Air & Water Reactions
Water soluble.
Reactivity Profile
Actidione is an imide. Actidione is incompatible with strong oxidizing agents, acid chlorides and acid anhydrides. Actidione decomposes rapidly in alkali at room temperature.
Health Hazard
Actidione is extremely toxic; the probable oral lethal dose in humans is 5-50 mg/kg, or 7 drops to 1 teaspoonful for a 150-lb. person.
Fire Hazard
When exposed to heat, Actidione emits toxic fumes, including nitrogen oxides.
Agricultural Uses
Fungicide; plant growth regulator: A U.S. EPA restricted Use Pesticide (RUP). Used as an antibiotic, plant growth regulator, and protein synthesis inhibitor. Inhibits growth of many plant pathogenic fungi. Effective for control of powdery mildew on roses and many other ornamentals, rusts and leaf spots on lawn grasses, and azalea petal blight. Also used as a repellent for rodents and other animal pests and in cancer therapy. Not listed for use in EU countries
Trade name
ACTI-AID®[C]; ACTIDIONE®[C]; ACTIDIONE® TGF[C]; ACTIDONE®; ACTIDONE® PM; ACTIDONE® TGF; ACTISPRAY; HIZAROCIN®; KAKEN®; NARAMYCIN®; NARAMYCIN A®; NEOCYCLOHEXIMIDE®; U-4527
Biological Activity
Selective inhibitor of eukaryotic (over prokaryotic) protein synthesis, blocking tRNA binding and release from ribosomes. Induces apoptosis in a variety of transformed and normal cell lines, including T-cells. Competitively inhibits the PPIase hFKBP12 (K i = 3.4 μ M). Antifungal antibiotic.
Pharmacology
Strongly inhibits the growth of pathogenic fungi but no effects on bacterial growth, even at 100 mg/ml. Inhibits protein synthesis by interfering with the translocation step in eukaryotes, but not in prokaryotes. When ingested by animals, the agent causes excitement, tremors, salivation, diarrhea, and melena.
Potential Exposure
A potential danger to those involved in the manufacture, formulation, or application of this fun- gicide and pesticide. Used as an antibiotic, plant growth regulator, and protein synthesis inhibitor. Used on oranges for processing and to inhibit growth of many pathogenic plant fungi. Also used as a repellent for rodents and other animal pests; and in cancer therapy.
in vitro
cycloheximide blocks the movement of peptidyl-trna from acceptor site to the donor site on reticulocyte ribosomes. this translocation reaction is dependent on the transfer enzyme, tf-ii, and gtp hydrolysis. cycloheximide has no effect on the ribosome dependent gtpase activity of tf-ii or peptidyl transferase reaction by which peptides on trna in the donor ribosomal site are transferred to an amino acid on trna in the acceptor site [1].
in vivo
cycloheximide treatment was effective in attenuating rat brain injury within a 6 hr therapeutic window after hypoxia-ischemia in a newborn rat pup model. these data support the possibility that protein synthesis inhibitors, as well as other anti-apoptotic strategies, may have therapeutic utility in hypoxic-ischemic (hi) events of the developing newborn brain even when treatment is delayed for up to 6 hr after the primary asphyxial insult [2].
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h following skin contact.
Carcinogenicity
Cycloheximide is genotoxic in Escherichia coli with metabolic activation and in the mouse sperm morphology assay. Carcinogenicity bioassays in the mouse and rat are inconclusive.
Environmental Fate
CHX is a potent inhibitor of protein synthesis in animals. It binds to E-site of 70S ribosome-mRNA complex, blocking the translational step of protein biosynthesis. It causes an increase in adrenal RNA and increased production of glucocorticoids.
Metabolism
Rapidly inactivated at room temperature by diluted alkali with the formation of a volatile, fragrant ketone, 2,4- dimethylcyclohexanone. Hazardous to fish and wildlife.
storage
room temperature
Purification Methods
It crystallises from H2O /MeOH (4:1), amyl acetate, isopropyl acetate/isopropyl ether or H2O. [Beilstein 21/13 V 434.]
Toxicity evaluation
Skin irritant. LD50 2 mg/kg (rat, orl);
133 mg/kg (mice, Ipr); 65 mg/kg (guinea pig); 60 mg/kg
(monkey). Teratogenic effects.
To remove toxicant
from gut, activated charcoal and a catharitic dose of sodium sulfate are effective. Mechanisms of toxicity
are not well defined, but hydrocortisone is antidotal,
particularly in combination with the adrenergic agent
methoxyphenamine.
Incompatibilities
Incompatible with oxidizers, acid anhy- drides; strong bases.
Waste Disposal
High-temperature incinerator with flue gas scrubbing equipment.
References
1) Merck 14:2728
Properties of Actidione
| Melting point: | 111-116 °C |
| Boiling point: | 492℃ |
| alpha | -28.5 º (c=1, CHCl3) |
| Density | 1.0867 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| Flash point: | 87°0°(230°F) |
| storage temp. | 2-8°C |
| solubility | Soluble in Ethanol (up to 14 mg/ml) or in Water (up to 7 mg/ml). |
| form | Glassy Irregular Shaped Granules |
| pka | 11.61±0.40(Predicted) |
| color | White |
| Water Solubility | 2.1 g/100 mL (2 ºC) |
| BRN | 88868 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, acid chlorides, acid anhydrides, alkali. |
| CAS DataBase Reference | 66-81-9(CAS DataBase Reference) |
| EPA Substance Registry System | Cycloheximide (66-81-9) |
Safety information for Actidione
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H341:Germ cell mutagenicity H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Actidione
Related products of tetrahydrofuran






You may like
-
 Cycloheximide for tissue culture CAS 66-81-9View Details
Cycloheximide for tissue culture CAS 66-81-9View Details
66-81-9 -
 Cycloheximide extrapure CAS 66-81-9View Details
Cycloheximide extrapure CAS 66-81-9View Details
66-81-9 -
 Actidione, GR 98% CAS 66-81-9View Details
Actidione, GR 98% CAS 66-81-9View Details
66-81-9 -
 Actidione, for tissue culture CAS 66-81-9View Details
Actidione, for tissue culture CAS 66-81-9View Details
66-81-9 -
 Actidione 98% (HPLC) CAS 66-81-9View Details
Actidione 98% (HPLC) CAS 66-81-9View Details
66-81-9 -
 Cycloheximide CAS 66-81-9View Details
Cycloheximide CAS 66-81-9View Details
66-81-9 -
 ACTIDIONE AR CAS 66-81-9View Details
ACTIDIONE AR CAS 66-81-9View Details
66-81-9 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6