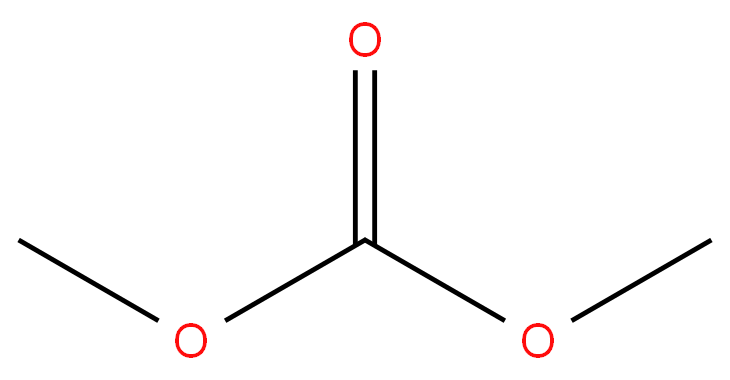
Dimethyl carbonate
Synonym(s):Carbonic acid dimethyl ester;Dimethyl carbonate;dimethyl ester;DMC;DMC, Methyl carbonate, Carbonic acid dimethyl ester
- CAS NO.:616-38-6
- Empirical Formula: C3H6O3
- Molecular Weight: 90.08
- MDL number: MFCD00008420
- EINECS: 210-478-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:35
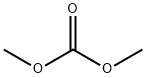
What is Dimethyl carbonate?
Description
Dimethyl carbonate is a colorless flammable liquid that was originally made by the reaction of 2 moles of methanol with 1 mole of phosgene. But because of phosgene''s extreme toxicity, it is now manufactured by the transesterification of propylene carbonate and methanol or the copper-catalyzed reaction of methanol, oxygen, and carbon monoxide. Dimethyl carbonate is increasingly used as a?methylating agent in place of hazardous, waste-generating compounds, such as dimethyl sulfate and methyl halides.
Chemical properties
Dimethyl carbonate is miscible with most acids and alkalis, soluble in most organic solvents, but insoluble in water.
The Uses of Dimethyl carbonate
Dimethyl carbonate is used as a solvent in organic synthesis and considered as a replacement for solvent like methyl ethyl ketone, tert-butyl acetate and parachlorobenzotrifluoride. It is involved as an intermediate in the preparation of diphenylcarbonate, which in turn is used as a key raw material for the synthesis of Bisphenol-A-polycarbonate. It is also used as a 'green' methylating agent involved in the methylation of aniline, phenols and carboxylic acids. It can be used as a fuel additive due to its high oxygen content. It also finds applications related to supercapacitors and lithium batteries.
The Uses of Dimethyl carbonate
Environmentally benign substitute for dimethyl sulfate, q.v., and methyl halides in methylation reactions and for phosgene, q.v., in methylcarbonylation reactions.
Definition
ChEBI: Dimethyl carbonate is a carbonate ester that is carbonic acid in which both hydrogens are replaced by methyl groups. A flammable, colourless liquid (m.p. 2-4°C, b.p. 90°C) with a characterstic ester-like odour, it is used as a 'green' methylating agent and as a solvent. It has a role as a solvent and a reagent.
Preparation
Dimethyl carbonate (DMC) is formed by the reaction of MeOH with phosgene or methyl chloroformate in the presence of a concentrated sodium hydroxide solution in a two-phase reaction in high yields and purity. Other alcohols can also be phosgenated. As DMC is now more easily accessible via the direct oxidative carbonylation of MeOH, phosgenation is losing its attractiveness in this application.
Preparation
Dimethyl carbonate is traditionally prepared by the reaction of phosgene and methanol. Methyl chloroformate is produced as an intermediate:
COCl2 + CH3OH → CH3OCOCl + HCl
CH3OCOCl + CH3OH → CH3OCO2CH3 + HCl
Safety
Dimethyl carbonate(DMC) is a flammable liquid with a flash point of 17 °C (63 °F), which limits its use in consumer and indoor applications. DMC is metabolized by the body to methanol and carbon dioxide, so accidental ingestion should be treated in the same manner as methanol poisoning
Synthesis Reference(s)
The Journal of Organic Chemistry, 49, p. 1122, 1984 DOI: 10.1021/jo00180a033
Tetrahedron Letters, 15, p. 803, 1974
General Description
Dimethyl carbonate appears as a clear, colorless liquid with a pleasant odor. Denser than water and slightly soluble in water. Vapors are heavier than air. Used to make other chemicals and as a special purpose solvent.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Dimethyl carbonate reacts with acids to liberate heat along with methanol and carbon dioxide. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction with caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides.
Hazard
Flammable, dangerous fire risk. Toxic by inhalation, strong irritant.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Highly flammable
Safety Profile
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. An irritant. Violent reaction or ignition on contact with potassium-tertbutoxide. A very dangerous fire hazard when exposed to heat, open flames (sparks), or oxiduers. To fight fire, use alcohol foam. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
If the reagent has broad intense bands at 3300cm-1 and above (i.e. OH stretching), then it should be purified further. Wash it successively with 10% Na2CO3 solution, saturated CaCl2, H2O, and dry it by shaking mechanically for 1hour with anhydrous CaCl2, and fractionate. [Bowden & Butler J Chem Soc 78 1939, Vogel J Chem Soc 1847 1948, Beilstein 3 IV 3.]
Properties of Dimethyl carbonate
| Melting point: | 2-4 °C (lit.) |
| Boiling point: | 90 °C (lit.) |
| Density | 1.069 g/mL at 25 °C (lit.) |
| vapor density | 3.1 (vs air) |
| vapor pressure | 18 mm Hg ( 21.1 °C) |
| refractive index | n |
| Flash point: | 65 °F |
| storage temp. | Store below +30°C. |
| solubility | 139g/l |
| form | Liquid |
| color | <50(APHA) |
| Odor | Pleasant |
| explosive limit | 4.22-12.87%(V) |
| Water Solubility | 139 g/L |
| Sensitive | Moisture Sensitive |
| Merck | 14,3241 |
| BRN | 635821 |
| Dielectric constant | 3.1699999999999999 |
| Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents, potassium t-butoxide. |
| CAS DataBase Reference | 616-38-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Carbonic acid, dimethyl ester(616-38-6) |
| EPA Substance Registry System | Carbonic acid, dimethyl ester (616-38-6) |
Safety information for Dimethyl carbonate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for Dimethyl carbonate
| InChIKey | IEJIGPNLZYLLBP-UHFFFAOYSA-N |
Dimethyl carbonate manufacturer
JSK Chemicals
Manav Biochem Impex Private Limited
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Dimethyl Carbonate 99%View Details
Dimethyl Carbonate 99%View Details -
 Dimethyl carbonate 99%View Details
Dimethyl carbonate 99%View Details -
 Dimethyl Carbonate (DMC) 99%View Details
Dimethyl Carbonate (DMC) 99%View Details -
 DI METHYL CARBONATE 99%View Details
DI METHYL CARBONATE 99%View Details -
 Dimethyl carbonate 98%View Details
Dimethyl carbonate 98%View Details -
 Di Methyl Carbonate CASView Details
Di Methyl Carbonate CASView Details -
 Dimethyl Carbonate CASView Details
Dimethyl Carbonate CASView Details -
 Dimethyl Carbonate CASView Details
Dimethyl Carbonate CASView Details
