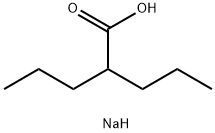
2-Propylpentanoic acid
Synonym(s):2-Propylpentanoic acid;2-Propylvaleric acid;Dipropylacetic acid, Valproic acid, 2-Propylpentanoic acid;Valproic acid
- CAS NO.:99-66-1
- Empirical Formula: C8H16O2
- Molecular Weight: 144.21
- MDL number: MFCD00002672
- EINECS: 202-777-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
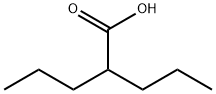
What is 2-Propylpentanoic acid?
Absorption
The intravenous and oral forms of valproic acid are expected to produce the same AUC, Cmax, and Cmin at steady-state. The oral delayed-release tablet formulation has a Tmax of 4 hours. Differences in absorption rate are expected from other formulations but are not considered to be clinically important in the context of chronic therapy beyond impacting frequency of dosing. Differences in absorption may create earlier Tmax or higher Cmax values on initiation of therapy and may be affected differently by meals. The extended release tablet formulation had Tmax increase from 4 hours to 8 hours when taken with food. In comparison, the sprinkle capsule formulation had Tmax increase from 3.3 hours to 4.8 hours. Bioavailability is reported to be approximately 90% with all oral formulations with enteric-coated forms possibly reaching 100%.
Toxicity
LD50 Values
Oral, mouse: 1098 mg/kg
Oral, rat: 670 mg/kg
Overdose
Symptoms of overdose include somnolence, heart block, deep coma, and hypernatremia. Fatalities have been reported, however patients have recovered from valproate serum concentrations as high as 2120 mcg/mL. The unbound fraction may be removed by hemodialysis. Naloxone has been demonstrated to reverse the CNS depressant effects of overdose but may also reverse the anti-epileptic effects.
Reproductive Toxicity
Valproate use in pregnancy is known to increase the risk of neural tube defects and other structural abnormalities. The risk of spina bifida increases from 0.06-0.07% in the normal population to 1-2% in valproate users. The North American Antiepileptic Drug (NAAED)
Pregnancy Registry reports a major malformation rate of 9-11%, 5 times the baseline rate. These malformations include neural tube defects, cardiovascular malformations, craniofacial defects (e.g., oral clefts, craniosynostosis), hypospadias, limb malformations (e.g., clubfoot, polydactyly), and other malformations of varying severity involving other body systems. Other antiepileptic drugs, lamotrigine, carbemazepine, and phenytoin, have been found to reduce IQ in children exposed in utero. Valproate was also studied however the results did not achieve statistical significance (97 IQ (CI: 94-101)). Observational studies report an absolute risk increase of 2.9% (relative risk 2.9 times baseline) of autism spectrum disorder in children exposed to valproate in utero. There have been case reports of fatal hepatic failure in children of mothers who used valproate during pregnancy.
There have been reports of male infertility when taking valproate.
Lactation
Valproate is excreted in human milk. Data in the published literature describe the presence of valproate in human milk (range: 0.4 mcg/mL to 3.9 mcg/mL), corresponding to 1% to 10% of maternal serum levels. Valproate serum concentrations collected from breastfed infants aged 3 days postnatal to 12 weeks following delivery ranged from 0.7 mcg/mL to 4 mcg/mL, which were 1% to 6% of maternal serum valproate levels. A published study in children up to six years of age did not report adverse developmental or cognitive effects following exposure to valproate
via breast milk.
Other Toxicity Considerations
Use in pediatrics under 2 years of age increases the risk of fatal hepatotoxicity.
Description
Valproic acid and its salts are a new group of antiepileptic drugs that differs from the
known drugs both structurally and in terms of its mechanism of action. It is believed that
it acts on the metabolism of the GABA system. Valproic acid has been shown to elevate
the level of GABA in the brain by means of competitive inhibition of GABA transaminase
and the dehydrogenase of succinic semialdehyde.
This drug not only exhibits anticonvulsant action, but also betters the mental condition
of the patient.
Description
Valproic acid (VPA), formally 2-propylpentanoic acid, is a low–molecular weight branched-chain fatty acid.?It?is a derivative?of valeric (pentanoic) acid1, which occurs naturally in valerian (Valeriana officinalis), an Eastern Hemisphere flowering plant.
VPA has been known since at least the 1880s, when it was synthesized by American chemist Beverly S. Burton. In 1915, a monumental work on optically active fatty acids by Emil Abderhagen and Egon Eichwald at the University of Halle (Germany) reported that VPA was optically active, even though an inspection of its structure shows that that cannot be true.
VPA is widely used for treating several diseases, including epilepsy and bipolar disorder. Until the 1960s, it was used only as a solvent for pharmaceuticals and some industrial chemicals; but in 1963, George Carraz and colleagues at Laboratoire Berthier (Grenoble, France) discovered that all of the compounds they were testing for anticonvulsant activity gave positive results when they were dissolved in VPA. This serendipitous finding led to France’s approval of VPA for treating epilepsy in 1967. The US Food and Drug Administration granted similar approval in 1978.
VPA is well known for inhibiting histone deacetylases, enzymes that allow histone proteins to wrap DNA more tightly than they otherwise would. It also has the ability to reduce the stability of certain proteins, including Cas9 (CRISPR-associated protein 9), which plays an important role in prokaryotic adaptive immunity. Its precise mechanism-of-action for destabilizing Cas9, however, is still largely unknown.
Chemical properties
Colorless Liquid
Originator
Depakote,Abbott
The Uses of 2-Propylpentanoic acid
2-Propylpentanoic acid has been used as a supplement in mouse embryonic fibroblast - conditioned medium (MEF-CM)?to feed the cells.
The Uses of 2-Propylpentanoic acid
Antiepileptic; increases levels of -aminobutyric acid(GABA) in the brain. Anticonvulsant that also has efficacy as a mood stabilizer in bipolar disorder
The Uses of 2-Propylpentanoic acid
Antiepileptic; Anticonvulsant that also acts as a mood stabilizer for those with bipolar disorder.
The Uses of 2-Propylpentanoic acid
For treatment and management of seizure disorders, mania, and prophylactic treatment of migraine headache. In epileptics, valproic acid is used to control absence seizures, tonic-clonic seizures (grand mal), complex partial seizures, and the seizures asso
What are the applications of Application
Valproic Acid is A branched chain fatty acid
Indications
Indicated for:
1) Use as monotherapy or adjunctive therapy in the management of complex partial seizures and simple or complex absence seizures.
2) Adjunctive therapy in the management of multiple seizure types that include absence seizures.
3) Prophylaxis of migraine headaches.
4) Acute management of mania associated with bipolar disorder.
Off-label uses include:
1) Maintenance therapy for bipolar disorder.
2) Treatment for acute bipolar depression.
3) Emergency treatment of status epilepticus.
Background
Valproic acid, or valproate, is an fatty acid derivative and anticonvulsant originally synthesized in 1881 by Beverly S. Burton. It enjoyed use as a popular organic solvent in industry and pharmaceutical manufacturing for nearly a century. In 1963, a serendipitous discovery was made by George Carraz during his investigations into the anticonvulsant effects of khelline when he found that all of his samples, dissolved in valproic acid, exerted a similar degree of anticonvulsive activity. It first received approval on February 28, 1978 from the FDA under the trade name Depakene.
Since then, it has been investigated for neuroprotective, anti-manic, and anti-migraine effects. It is currently a compound of interest in the field of oncology for its anti-proliferative effects and is the subject of many clinical trials in a variety of cancer types.
Definition
ChEBI: A branched-chain saturated fatty acid that comprises of a propyl substituent on a pentanoic acid stem.
Manufacturing Process
Dipropyl acetic acid or valproic acid may be prepared the next way.
Propylbromide is mixed with cyanacetic acid in the presence of sodium
ethylate, made from absolute ethanol and sodium. By that prepared α,α-
dipropylcyanacetic acid ethyl ester is saponified with equimolecular amounts
of NaOH to give dipropylacetonitril. The desired dipropylacetic acid is produced
by saponification of dipropylacetonitryl with aquatic NaOH. It is colorless
liquid. BP 219°-220°C.
Sodium salt of this acid may be prepared by adding of equivalent of NaOH.
brand name
Depakene (Abbott);Valproine;Vederon.
Therapeutic Function
Anticonvulsant
Biological Functions
Although it is marketed as both valproic acid
(Depakene) and as sodium valproate (Depakote), it is
the valproate ion that is absorbed from the gastrointestinal
tract and is the active form.
As with several other AEDs, it is difficult to ascribe
a single mechanism of action to valproic acid.This compound
has broad anticonvulsant activity, both in experimental
studies and in the therapeutic management of
human epilepsy.Valproic acid has been shown to block
voltage-dependent sodium channels at therapeutically
relevant concentrations. In several experimental studies,
valproate caused an increase in brain GABA; the
mechanism was unclear.There is evidence that valproate may also inhibit T-calcium channels and that this may
be important in its mechanism of action in patients with
absence epilepsy.
General Description
Clear colorless liquid.
General Description
VPA is an established AED with a simple chemical structurebut an unusually broad spectrum of action. It is generallywell tolerated, but its use is limited by two rare but significanttoxic side effects (hepatotoxicity and teratogenicity) thatcan be dose-dependent or idiosyncratic in nature.Thesedrawbacks are apparently shared by its equipotent activemetabolite, (E)-2-propyl-2-pentenoic acid (2-ene-VPA).
VPA is also an important inhibitor of the cytochrome P450isozymes, mainly of CYP2C9 and also of uridine diphosphate(UDP)-glucuronyl transferase and epoxide hydrolase.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2-Propylpentanoic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 2-Propylpentanoic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. 2-Propylpentanoic acid is incompatible with bases, oxidizing agents and reducing agents. 2-Propylpentanoic acid is corrosive. .
Fire Hazard
2-Propylpentanoic acid is combustible.
Biochem/physiol Actions
Anticonvulsant that also has efficacy as a mood stabilizer in bipolar disorder
Mechanism of action
Although its mechanism of action is not clearly established, valproate appears to increase the inhibitory effect of GABA,
possibly by activation of glutamic acid decarboxylase or inhibition of GABA-transaminase). The high drug
concentrations required, however, cast doubt on the clinical relevance of this effect. Furthermore, valproate recently has been
shown to decrease the uptake of GABA into cultured astrocytes; this action may contribute to the AED efficacy. Valproate
is known to produce a blockade of high-frequency repetitive firing by slowing the rate of Na+
recovery from inactivation, a
mechanism consistent with the actions of phenytoin and CBZ. Valproate blocks the low-threshold T-type Ca2+ channel.
Consequently, the overall therapeutic utility of valproate is likely caused by multiple effects.
Valproate is indicated for initial or adjunct treatment of absence seizures or as an adjunct when absence seizures occur in
combination with either tonic-clonic seizures, myoclonic seizures, or both. For patients with unambiguous idiopathic generalized
epilepsy, valproate often is the drug of choice, because it controls absence, myoclonic, and generalized tonic-clonic seizures
well. It also is approved by U.S. FDA for use in complex partial seizures, occurring with or without other seizure types in
adults or children 10 years of age or older. In new patients with typical absence seizures, ethosuximide is preferred to
valproate because of the latter drug's risk of producing hepatotoxicity. In a comparative trial, sodium valproate and
ethosuximide were equally effective when either drug was given alone or in combination with other AEDs in children with typical
absence seizures. In atypical absence seizures (Lennox-Gastaut syndrome), sodium valproate is more effective, whereas in
myoclonic seizures, it is less effective than clonazepam. Valproate is approved by the U.S. FDA for use in bipolar disorder and
against migraine headaches.
Pharmacokinetics
Valproate has been shown to reduce the incidence of complex partial seizures and migraine headaches. It also improves symptom control in bipolar mania. Although the exact mechanisms responsible are unknown, it is thought that valproate produces increased cortical inhibition to contribute to control of neural synchrony. It is also thought that valproate exerts a neuroprotective effect preventing damage and neural degeneration in epilepsy, migraines, and bipolar disorder.
Valproate is hepatotoxic and teratogenic. The reasons for this are unclear but have been attributed to the genomic effects of the drug.
A small proof-of concept study found that valproate increases clearance of human immunodeficiency virus (HIV) when combined with highly active antiretroviral therapy (HAART) by reactivating the virus to allow clearance, however, a larger multicentre trial failed to show a significant effect on HIV reservoirs when added to HAART. The FDA labeling contains a warning regarding HIV reactivation during valproate use..
Pharmacokinetics
Valproate undergoes rapid and complete absorption, which is only slightly slowed by food. It is 90% protein bound, and its clearance is dose-dependent because of an increase in the free fraction of the drug at higher doses. It is metabolized almost entirely by the liver, with 30 to 50% of an orally administered dose being eliminated in the urine as its acyl glucuronide conjugate, 40% from mitochondrial β-oxidation, approximately 15 to 20% by ω-oxidation, and less than 3% is excreted unchanged in urine. Its major active metabolite is (E)-2-ene valproate (trans 2-ene valproate). Its 4-ene metabolite has been proposed to be a reactive metabolite responsible for the hepatotoxicity of valproate. Other metabolites found in the urine include 3-oxo- and 4-hydroxyvalproate. The elimination half-life for valproate ranged from 9 to 16 hours following oral dosing regimens of 250 to 1,000 mg. Patients who are not taking enzyme-inducing AEDs (carbamazepine, phenytoin, and phenobarbital) will clear valproate more rapidly; therefore, monitoring of AED plasma concentrations should be intensified whenever concurrent AEDs are introduced or withdrawn.
Clinical Use
Valproic acid is well absorbed from the gastrointestinal
tract and is highly bound (~90%) to plasma protein,
and most of the compound is therefore retained
within the vascular compartment.Valproate rapidly enters
the brain from the circulation; the subsequent decline
in brain concentration parallels that in plasma, indicating
equilibration between brain and capillary
blood. A large number of metabolites have been identified,
but it is not known whether they play a role in the
anticonvulsant effect of the parent drug. Valproic acid
inhibits the metabolism of several drugs, including phenobarbital,
primidone, carbamazepine, and phenytoin,
leading to an increased blood level of these compounds.
At high doses, valproic acid can inhibit its own metabolism.
It can also displace phenytoin from binding sites
on plasma proteins, with a resultant increase in unbound
phenytoin and increased phenytoin toxicity. In
this instance, the dosage of phenytoin should be adjusted
as required. These examples reinforce the need
to determine serum anticonvulsant levels in epileptic
patients when polytherapy is employed.
Valproic acid has become a major AED against several
seizure types. It is highly effective against absence
seizures and myoclonic seizures. In addition, valproic
acid can be used either alone or in combination with
other drugs for the treatment of generalized tonic–
clonic epilepsy and for partial seizures with complex
symptoms.
Side Effects
The most serious adverse effect associated with valproic
acid is fatal hepatic failure. Fatal hepatotoxicity is
most likely to occur in children under age 2 years, especially
in those with severe seizures who are given multiple
anticonvulsant drug therapy. The hepatotoxicity is
not dose related and is considered an idiosyncratic reaction;
it can occur in individuals in other age groups,
and therefore, valproic acid should not be administered
to patients with hepatic disease or significant hepatic
dysfunction or to those who are hypersensitive to it.
Valproic acid administration has been linked to an increased
incidence of neural tube defects in the fetus of
mothers who received valproate during the first
trimester of pregnancy. Patients taking valproate may
develop clotting abnormalities.
Valproic acid causes hair loss in about 5% of patients,
but this effect is reversible. Transient gastrointestinal
effects are common, and some mild behavioral
effects have been reported. Metabolic effects, including
hyperglycemia, hyperglycinuria, and hyperammonemia,
have been reported. An increase in body weight also
has been noted. Valproic acid is not a CNS depressant,
but its administration may lead to increased depression
if it is used in combination with phenobarbital, primidone,
benzodiazepines, or other CNS depressant agents.
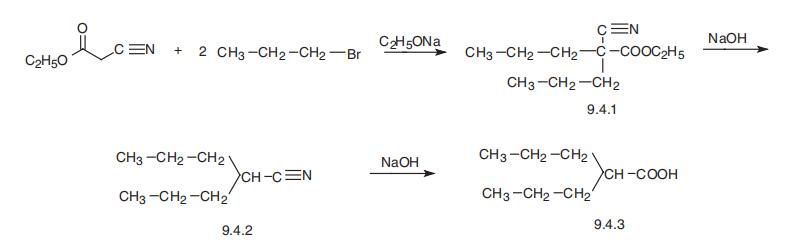
Synthesis
Valproic acid, 2-propylvaleric acid (9.4.3), is synthesized by the alkylation of cyanoacetic ester with two moles of propylbromide, to give dipropylcyanoacetic ester (9.4.1). Hydrolysis and decarboxylation of the carbethyoxy group gives dipropylacetonitrile (9.4.2), which is hydrolyzed into valproic acid (9.4.3) [12¨C15].
Metabolism
Most drug is metabolized to glucuronide conjugates (30-50%) of the parent drug or of metabolites. Another large portion is metabolized through mitochondrial β-oxidation (40%). The remainder of metabolism (15-20%) occurs through oxidation, hydroxylation, and dehydrogenation at the ω, ω1, and ω2 positions resulting in the formation of hydroxyls, ketones, carboxyls, a lactone metabolite, double bonds, and combinations.
Solubility in organics
soluble in most organic solvents, including methanol, chloroform, and ether, solubility in water: 1.27 mg/mL.
References
[1] phiel c j, zhang f, huang e y, et al. histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. journal of biological chemistry, 2001, 276(39): 36734-36741.
[2] chateauvieux s, morceau f, dicato m, et al. molecular and therapeutic potential and toxicity of valproic acid. biomed research international, 2010, 2010.
Properties of 2-Propylpentanoic acid
| Melting point: | -21.25°C (estimate) |
| Boiling point: | 220 °C (lit.) |
| Density | 0.9 g/mL at 25 °C (lit.) |
| vapor pressure | 0.01 hPa (20 °C) |
| refractive index | n |
| Flash point: | 232 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: slightly soluble |
| appearance | colorless liquid |
| form | Liquid |
| pka | 4.6(at 25℃) |
| color | Clear colorless to pale yellow |
| Odor Threshold | 0.0033ppm |
| explosive limit | 1%(V) |
| Water Solubility | slightly soluble |
| Merck | 14,9913 |
| BRN | 1750447 |
| CAS DataBase Reference | 99-66-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Valproic Acid(99-66-1) |
| EPA Substance Registry System | Valproic acid (99-66-1) |
Safety information for 2-Propylpentanoic acid
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 2-Propylpentanoic acid
2-Propylpentanoic acid manufacturer
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Valproic Acid 99%View Details
Valproic Acid 99%View Details -
 Valproic acid 99-66-1 98%View Details
Valproic acid 99-66-1 98%View Details
99-66-1 -
 99-66-1 99%View Details
99-66-1 99%View Details
99-66-1 -
 2-Propylvaleric Acid CAS 99-66-1View Details
2-Propylvaleric Acid CAS 99-66-1View Details
99-66-1 -
 Valproic Acid CASView Details
Valproic Acid CASView Details -
 2-Propylvaleric acid CAS 99-66-1View Details
2-Propylvaleric acid CAS 99-66-1View Details
99-66-1 -
 2-Propylpentanoic acid CAS 99-66-1View Details
2-Propylpentanoic acid CAS 99-66-1View Details
99-66-1 -
 Valproic Acid Api, Purity: 99%, CAS Number: 99-66-1View Details
Valproic Acid Api, Purity: 99%, CAS Number: 99-66-1View Details
99-66-1
