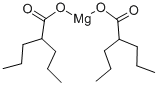
Divalproex sodium
- CAS NO.:76584-70-8
- Empirical Formula: C8H17NaO2
- Molecular Weight: 168.21
- MDL number: MFCD00879852
- EINECS: 202-303-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
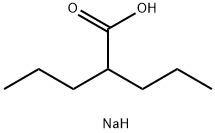
What is Divalproex sodium?
Description
Divalproex is a prodrug form of valproic acid that contains valproic acid and valproate sodium. Formulations containing divalproex exhibit delayed gastrointestinal absorption and are converted to valproic acid in the intestine, which reduces gastric irritation and nervous system side effects. Formulations containing divalproex are widely used for the treatment of seizures and bipolar disorder.
Chemical properties
White Solid
Originator
Depakote,Abbott
The Uses of Divalproex sodium
Divalproex sodium consists of a compound of sodium valproate and valproic acid in a 1:1 molar relationship in an enteric coated form. In rare cases, it is also used as a treatment for major depressive disorder, and increasingly taken long-term for prevent
The Uses of Divalproex sodium
Antiepileptic; Anticonvulsant that also acts as a mood stabilizer for those with bipolar disorder.
The Uses of Divalproex sodium
Anticonvulsant; Bipolar Agent
What are the applications of Application
Valproic Acid Semisodium Salt is an antiepileptic and anticonvulsant substance
Definition
ChEBI: A mixture of valproic acid and its sodium salt in a 1:1 molar ratio. It is used for the management and treatment of seizure disorders, mania, and prophylactic treatment of migraine headache.
Manufacturing Process
Dipropyl acetic acid or valproic acid may be prepared the next way.
Propylbromide is mixed with cyanacetic acid in the presence of sodium
ethylate, made from absolute ethanol and sodium. By that prepared α,α-
dipropylcyanacetic acid ethyl ester is saponified with equimolecular amounts
of NaOH to give dipropylacetonitril. The desired dipropylacetic acid is produced
by saponification of dipropylacetonitryl with aquatic NaOH. It is colorless
liquid. BP 219°-220°C.
Sodium salt of this acid may be prepared by adding of equivalent of NaOH.
brand name
Depakote (Abbott).
Therapeutic Function
Anticonvulsant
Clinical Use
Treatment of manic episodes associated with bipolar disorder
Migraine prophylaxis (unlicensed)
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism possibly inhibited
by erythromycin; avoid with pivmecillinam;
concentration reduced by carbapenems - avoid.
Antidepressants: avoid with St John’s wort.
Antiepileptics: concentration reduced by
carbamazepine; concentration of active
carbamazepine metabolite increased; increased
concentration of lamotrigine, phenobarbital,
rufinamide and possibly ethosuximide; sometimes
reduces concentration of active metabolite of
oxcarbazepine; alters phenytoin concentration;
phenytoin and phenobarbital reduce valproate
concentration; hyperammonaemia and CNS toxicity
with topiramate.
Antipsychotics: increased neutropenia with
olanzapine; possibly increases or decreases
concentration of clozapine; possibly increases
quetiapine concentration.
Ciclosporin: variable ciclosporin blood level response.
Sodium oxybate: concentration of sodium oxybate
increased.
Ulcer-healing drugs: metabolism inhibited by
cimetidine, increased concentration.
Metabolism
Valproic acid is extensively metabolised in the liver, a large
part by glucuronidation (up to 60
%) and the rest by a
variety of complex pathways (up to 45
%). It is excreted in
the urine almost entirely in the form of its metabolites;
small amounts are excreted in faeces and expired air.
Properties of Divalproex sodium
| Melting point: | 222 °C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White |
| CAS DataBase Reference | 76584-70-8(CAS DataBase Reference) |
Safety information for Divalproex sodium
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Divalproex sodium
Divalproex sodium manufacturer
Chynops Pharma
Sekhment pharmaventures
Roaq Chemicals Pvt Ltd
Mascot Industries Pvt., Ltd.
Ishita Active Pharma Ingredients Pvt. Ltd
Yogi Enterprise
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 76584-70-8 Divalproex Sodium 98%View Details
76584-70-8 Divalproex Sodium 98%View Details
76584-70-8 -
 Divalproex Sodium 98.10View Details
Divalproex Sodium 98.10View Details
76584-70-8 -
 Divalproex Sodium 99%View Details
Divalproex Sodium 99%View Details -
 76584-70-8 Divalproex sodium 98%View Details
76584-70-8 Divalproex sodium 98%View Details
76584-70-8 -
 Divalproex sodium 99%View Details
Divalproex sodium 99%View Details
76584-70-8 -
 Divalproex sodium 99%View Details
Divalproex sodium 99%View Details
76584-70-8 -
 Divalproex sodium 98% (HPLC) CAS 76584-70-8View Details
Divalproex sodium 98% (HPLC) CAS 76584-70-8View Details
76584-70-8 -
 Divalproex sodium CAS 76584-70-8View Details
Divalproex sodium CAS 76584-70-8View Details
76584-70-8