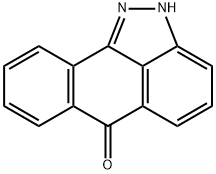
1,9-Pyrazoloanthrone
Synonym(s):1,9-Pyrazoloanthrone;Anthrapyrazolone;JNK Inhibitor II - CAS 129-56-6 - Calbiochem;SAPK Inhibitor II, Anthra[1,9- cd]pyrazol-6(2 H)-one, 1,9-pyrazoloanthrone, SP600125
- CAS NO.:129-56-6
- Empirical Formula: C14H8N2O
- Molecular Weight: 220.23
- MDL number: MFCD00022289
- EINECS: 204-955-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 12:42:02
What is 1,9-Pyrazoloanthrone?
Description
SP-600125 (129-56-6) is a selective inhibitor of c-Jun N-terminal kinase (JNK). Reversibly inhibits JNK1,2 and 3 (IC50‘s range from 40-90 nM). >300-fold selectivity for JNK as compared to related MAP kinases. Anti-inflammatory activity. SP-600125 inhibits expression of presenilin-1 and Notch signaling in mouse brain. Cell permeable and active in vivo.
The Uses of 1,9-Pyrazoloanthrone
A broad-spectrum serine/threonine kinase inhibitor of JNK with an IC50 range from 40 to 90 nM.
The Uses of 1,9-Pyrazoloanthrone
SP 600125 is a Jun N-terminal kinase (JNK) inhibitor.
The Uses of 1,9-Pyrazoloanthrone
C2C12 myoblasts were treated with SP600125 to test the stimulation of myogenesis.11 It was used to treat HepG2 cells to test the effects on oxysterol-induced necrosis.12
What are the applications of Application
SP600125 is a potent, selective and reversible inhibitor of JNK1, JNK2 and JNK3.
Definition
ChEBI: A member of the class of anthrapyrazoles that is anthra[1,9-cd]pyrazole substituted at position 6 by an oxo group. An inhibitor of c-Jun N-terminal kinase.
General Description
A potent, cell-permeable, selective, and reversible inhibitor of c-Jun N-terminal kinase (JNK) (IC50 = 40 nM for JNK-1 and JNK-2 and 90 nM for JNK-3). The inhibition is competitive with respect to ATP. Exhibits over 300-fold greater selectivity for JNK as compared to ERK1 and p38-2 MAP kinases. Inhibits the phosphorylation of c-Jun and blocks the expression of IL-2, IFN-γ, TNF-α, and COX-2 in cells. Blocks IL-1-induced accumulation of phospho-Jun and induction of c-Jun transcription.
Biological Activity
Selective inhibitor of c-Jun N-terminal kinase (JNK). Competitively and reversibly inhibits JNK1, 2 and 3 (IC 50 = 40-90 nM) with negligible activity at ERK2, p38 β and a range of enzymes (IC 50 > 10 μ M). Active in vivo . Shown to have reduced selectivity over other protein kinases under certain conditions. Protects renal tubular epithelial cells against ischemia/reperfusion-induced apoptosis. Also available as part of the MAPK Inhibitor Tocriset™ .
Biochem/physiol Actions
SP600125 is an anthrapyrazolone inhibitor of JNK that competes with ATP to inhibit the phosphorylation of c-Jun. It prevents the activation of inflammatory genes such as COX-2, IL-2 IFN-γ and TNF-α.8,9 It prevents the activation of JNK after brain ischemia and may be effective in treatment of ischemic stroke.10
Safety Profile
Poison by intravenous route.When heated to decomposition it emits toxic fumes ofNOx.
Synthesis
1,9-Pyrazolanthrone is prepared by diazotization of 1-aminoanthraquinone, reaction of the diazonium salt with sodium hydrogen sulfite, and cyclization of the resulting hydrazinosulfonic acid .
Storage
-20°C (desiccate)
References
1)Bennett et al. (2001), SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase; Proc. Natl. Acad. Sci. USA., 98 13681 2) Rahman et al. (2012), Intraperitoneal injection of JNK-specific inhibitor SP600125 inhibits the expression of presenilin-1 and Notch signaling in mouse brain without induction of apoptosis; Brain Res., 1448 117
Properties of 1,9-Pyrazoloanthrone
| Melting point: | 281~282℃ |
| Boiling point: | 361.16°C (rough estimate) |
| Density | 1.1702 (rough estimate) |
| refractive index | 1.5910 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: insoluble |
| form | Yellowish orange solid |
| pka | 11.75±0.20(Predicted) |
| color | yellow |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months |
| CAS DataBase Reference | 129-56-6(CAS DataBase Reference) |
| EPA Substance Registry System | Anthra[1,9-cd]pyrazol-6(2H)-one (129-56-6) |
Safety information for 1,9-Pyrazoloanthrone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for 1,9-Pyrazoloanthrone
| InChIKey | ACPOUJIDANTYHO-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
![Anthra[1,9-cd]pyrazol-6(2H)-one CAS 129-56-6](https://img.chemicalbook.in//Content/image/CP5.jpg) Anthra[1,9-cd]pyrazol-6(2H)-one CAS 129-56-6View Details
Anthra[1,9-cd]pyrazol-6(2H)-one CAS 129-56-6View Details
129-56-6 -
 SP600125 >98% (HPLC) CAS 129-56-6View Details
SP600125 >98% (HPLC) CAS 129-56-6View Details
129-56-6 -
 JNK Inhibitor II CASView Details
JNK Inhibitor II CASView Details -
 SP600125 CAS 129-56-6View Details
SP600125 CAS 129-56-6View Details
129-56-6 -
 JNK Inhibitor II CAS 129-56-6View Details
JNK Inhibitor II CAS 129-56-6View Details
129-56-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
