Pyraclostrobin
Synonym(s):Methyl {2-[1-(4-chlorophenyl)-1H-pyrazol-3-yloxymethyl]phenyl}methoxycarbamate
- CAS NO.:175013-18-0
- Empirical Formula: C19H18ClN3O4
- Molecular Weight: 387.82
- MDL number: MFCD03792774
- EINECS: 605-747-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
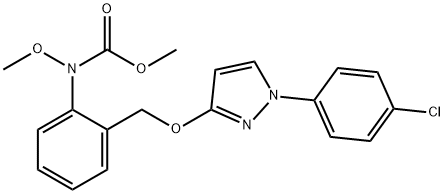
What is Pyraclostrobin?
The Uses of Pyraclostrobin
Pyraclostrobin is a fungicide for use in seed grass and food crops.
The Uses of Pyraclostrobin
Agricultural fungicide.
What are the applications of Application
Pyraclostrobin is a fungicide
Definition
ChEBI: Pyraclostrobin is a carbamate ester that is the methyl ester of [2-({[1-(4-chlorophenyl)-1H-pyrazol-3-yl]oxy}methyl)phenyl]methoxycarbamic acid. A fungicide used to control major plant pathogens including Septoria tritici, Puccinia spp. and Pyrenophora teres. It has a role as a mitochondrial cytochrome-bc1 complex inhibitor, a xenobiotic, an environmental contaminant and an antifungal agrochemical. It is a member of pyrazoles, a carbamate ester, an aromatic ether, a member of monochlorobenzenes, a methoxycarbanilate strobilurin antifungal agent and a carbanilate fungicide.
Agricultural Uses
Pyraclostrobin (trade names: Cabrio®, Headline®) primed the tobacco cv. Xanthi nc for more rapid accumulation of antimicrobial PR-1 defence proteins after infection with Tobacco mosaic virus and the wildfire pathogen Ps pv. tabaci. The Pyraclostrobininduced priming for enhanced PR-1 accumulation in response to pathogen attack was associated with enhanced disease resistance (Herms et al. 2002). The enhanced resistance to pathogenic viruses and bacteria in Pyraclostrobin-treated plants was also seen on various crops and ornamental plants in both the greenhouse and field (Koehle et al. 2003, 2006). It is interesting that in the field, Pyraclostrobin-induced priming was associated with enhanced resistance also to abiotic stresses, including drought. In addition, treatment with Pyraclostrobin increased crop yield in the field. Also, in various crops Pyraclostrobin and other strobilurin fungicides induce a ‘greening effect.’ The term refers to the phenomenon of delayed leaf senescence and an increased grain-filling period resulting in enhanced biomass and yield (Bartlett et al. 2002). Together, the findings made with Pyraclostrobin suggest that this chemistry, in addition to exerting direct antifungal activity, may also protect plants by priming them for a boosted activation of subsequently stress-induced defence responses. This conclusion is consistent with an earlier report demonstrating that another commercial fungicide, Oryzemate®, enhanced the resistance to Tobacco mosaic virus in tobacco (Koganezawa et al. 1998) and to a bacterial and an oomycete pathogen in Arabidopsis (Yoshioka et al. 2001). Oryzemate® contains Probenazole as the active ingredient which is metabolized to saccharin in treated plants (Koganezawa et al. 1998). The latter compound seems to elicit priming in Oryzemate®-treated plants (Siegrist et al. 1998).
Properties of Pyraclostrobin
| Melting point: | 63.7-65.2° |
| Boiling point: | 501.1±60.0 °C(Predicted) |
| Density | 1.27±0.1 g/cm3(Predicted) |
| storage temp. | 0-6°C |
| solubility | DMSO: 250 mg/mL (644.63 mM) |
| form | neat |
| pka | -0.23±0.10(Predicted) |
| form | Solid |
| color | Off-white to light yellow |
| Stability: | Toxic |
| NIST Chemistry Reference | Pyraclostrobine(175013-18-0) |
| EPA Substance Registry System | Pyraclostrobin (175013-18-0) |
Safety information for Pyraclostrobin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H331:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Pyraclostrobin
| InChIKey | HZRSNVGNWUDEFX-UHFFFAOYSA-N |
Pyraclostrobin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Pyraclostrobin 95.00% CAS 175013-18-0View Details
Pyraclostrobin 95.00% CAS 175013-18-0View Details
175013-18-0 -
 Pyraclostrobin CAS 175013-18-0View Details
Pyraclostrobin CAS 175013-18-0View Details
175013-18-0 -
 pyraclostrobin 10% cs Liquid Quinon (Pyraclostrobin 100 CS), 500 mLView Details
pyraclostrobin 10% cs Liquid Quinon (Pyraclostrobin 100 CS), 500 mLView Details
175013-18-0 -
 Pyraclostrobin 20 WgView Details
Pyraclostrobin 20 WgView Details
175013-18-0 -
 Liquid Pyraclostrobin 10% CS defender 1 LView Details
Liquid Pyraclostrobin 10% CS defender 1 LView Details
175013-18-0 -
 Liquid Pyraclostrobin 20 WG Fungicides, 1 LitreView Details
Liquid Pyraclostrobin 20 WG Fungicides, 1 LitreView Details
175013-18-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
