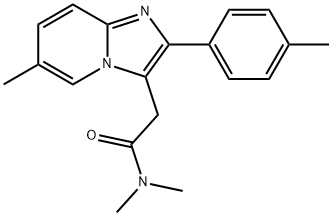
Zolpidem
Synonym(s):N,N,6-Trimethyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine-3-acetamide
- CAS NO.:82626-48-0
- Empirical Formula: C19H21N3O
- Molecular Weight: 307.39
- MDL number: MFCD00153885
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-20 16:56:21
What is Zolpidem?
Absorption
Zolpidem is rapidly absorbed from the gastrointestinal tract. In a single-dose crossover study in 45 healthy subjects given 5 and 10 mg zolpidem tartrate tablets, the average peak zolpidem concentrations (Cmax) were 59 and 121 ng/mL, respectively, occurring at a mean time (Tmax) of 1.6 hours for both doses .
Toxicity
Oral (male rat) LD50 = 695 mg/kg .
Overdose
Symptoms of overdose include impairment of consciousness ranging from somnolence to light coma, in addition to cardiorespiratory collapse resulting in fatal outcomes have been reported .
Withdrawal effects
Following rapid decreases in dose or abrupt discontinuation of zolpidem and other sedative/hypnotics, reports of signs and symptoms similar to those associated with withdrawal from other CNS-depressant drugs have been made .
Carcinogenesis
Zolpidem was administered to rats and mice over a span of 2 years at dietary dosages of 4, 18, and 80 mg/kg/day. In mice, these doses are considered 26 to 520 times or 2 to 35 times the maximum 10 mg human dose, respectively. In rats, these doses are 43 to 876 times or 6 to 115 times the maximum 10 mg human dose. No evidence of carcinogenicity was seen in mice. Renal liposarcomas were observed in 4/100 rats (3 males, 1 female) receiving 80 mg/kg/day, and a renal lipoma was observed in one male rat at the 18 mg/kg/day dose. Incidence rates of lipoma and liposarcoma for zolpidem were similar to those seen in historical control cases, and the tumor findings are presumed to be a spontaneous occurrence, not causally related to zolpidem .
Mutagenesis
Zolpidem did not show mutagenic activity in several tests including the Ames test, genotoxicity in mouse lymphoma cells in vitro, chromosomal aberrations in cultured human lymphocytes, abnormal DNA synthesis in rat hepatocytes in vitro, and the micronucleus test performed in mice .
Impairment of fertility
In a rat reproduction study, the high dose (100 mg base/kg) of zolpidem lead to irregular estrus cycles and prolonged precoital intervals, however, there was no effect on male or female fertility after daily oral doses comparable to 5 to 130 times the recommended human dose. No effects on any other fertility parameters were observed .
Use in pregnancy
This drug is considered a pregnancy category C drug. There are currently no sufficient conclusive studies completed in pregnant women to determine the safety of zolpidem use during pregnancy. Zolpidem should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus.
Use in nursing
From 0.004% to 0.019% of the total administered zolpidem dose is excreted into milk. The effect of zolpidem on the nursing infant is unknown at this time. Caution should be observed when zolpidem is administered to a nursing mother .
Description
Zolpidem hemitamwe is a non-benzodiazepine hypnotic with specific agonist activity at type 1 benzodiazepine receptors, and is indicated for use in insomnia and other sleep disorders. Structurally zolpidem belongs to a chemically distinct class, thus lacking the side-effects and abuse potential of classical benzodiazepines. It is currently being studied as a pre-operative sedative.
Description
Zolpidem—sold under a host of brand names, the best known of which is Ambien—is a medication used to treat insomnia. The article of commerce is the tartrate salt. In most people, it induces sleep rapidly and is effective for ≈2–3 hours.
The US patent on zolpidem was issued in 1984 to the French company Synthélabo, now part of Sanofi-Aventis. By 2007, it was off-patent; and the US Federal Drug Administration approved 13 generic versions of zolpidem tartrate.
Early in 2015, M. J. Miller and colleagues at the University of Notre Dame investigated whether?zolpidem could be used to treat tuberculosis. They found that zolpidem has some activity against?Mycobacterium tuberculosis?replication, but some of its structural isomers have much greater potency. The authors believe that "further optimization of the [zolpidem] core may well ''put TB to rest''."
Description
Zolpidem (Item No. 15792) is an analytical reference material that is functionally categorized as a sedative. It is a non-benzodiazepine drug that is used in the treatment of insomnia, and it has been recreationally abused. This product is intended for research and forensic applications.
Description
Zolpidem (Item No. 20648) is a certified reference material that is functionally categorized as a sedative. It is a non-benzodiazepine drug that is used in the treatment of insomnia, and it has been recreationally abused. This product is intended for research and forensic applications.
Chemical properties
Off-White Solid
Originator
Synthelabo (France)
The Uses of Zolpidem
A selective benzodiazepine receptor agonist not related chemically to benzodiazepines
The Uses of Zolpidem
A selective non-benzodiazepine GABAA receptor agonist. Sedative, hypnotic. Controlled substance (depresssant).
Background
Zolpidem, also known as Ambien, is a hypnotic drug that was initially approved by the FDA in 1992 . Zolpidem improves sleep in patients with insomnia. It is aimed for use in patients with difficulties initiating sleep. This drug decreases the time to fall asleep (sleep latency), increases the duration of sleep, and decreases the number of awakenings during sleep in patients with temporary (transient) insomnia. It is available in both immediate acting and extended release forms , .
Its tolerability profile is favorable when administered according to the manufacturer’s instructions, with a low risk of drug withdrawal, drug dependence, and drug tolerance . In addition, zolpidem improves sleep quality in patients suffering from chronic insomnia and can show mild muscle relaxant properties . Research also shows that zolpidem is rapid and effective in restoring brain function for patients in a vegetative state following brain injury. This drug has the propensity to completely or partially reverse the abnormal metabolism of damaged brain cells after injury , .
Indications
This drug is indicated for the short-term treatment of insomnia in adults characterized by difficulties with sleep initiation .
Definition
ChEBI: An imidazo[1,2-a]pyridine compound having a 4-tolyl group at the 2-position, an N,N-dimethylcarbamoylmethyl group at the 3-position and a methyl substituent at the 6-position.
brand name
Ambien (Sanofi Aventis);Stilnox.
General Description
Zolpidem (Ambien, an imidazopyridine) andeszopiclone (Lunesta, a cyclopyrrolone) are nonbenzodiazepinesand have been introduced as short- and moderate-acting hypnotics, respectively. Zolpidem exhibits ahigh selectivity for the α1 subunit of benzodiazepinebindingsite on GABAA receptor complex, whereas eszopicloneis a “superagonist” at BzRs with the subunitcomposition α1β2γ2 and α1β2γ3. Zolpidem has a rapidonset of action of 1.6 hours and good bioavailability(72%), mainly because it is lipophilic and has no ionizablegroups at physiological pH. Food can prolong the time topeak concentration without affecting the half-life probablyfor the same reason. It has short elimination half-life, becauseits aryl methyl groups is extensively α-hydroxylatedto inactive metabolites by CYP3A4 followed by furtheroxidation by aldehyde dehydrogenase to the ionic carboxylicacid. The metabolites are inactive, short-lived, andeliminated in the urine. Its half-life in the elderly or the patientswith liver disease is increased. Therefore, dosingshould be modified in patients with hepatic insufficiencyand the elderly. Because it has longer elimination half-lifethan zaleplon, it may be preferred for sleep maintenance.It was the most commonly prescribed drug for insomniain 2001.
Pharmacokinetics
Effects on the central nervous system (CNS)
This drug has CNS depressant effects, which may include somnolence, decreased alertness, sedation, drowsiness, dizziness, and other changes in psychomotor function . Due to the above effects, the FDA has recommended an initial dose of zolpidem (immediate-acting) is a single dose of 5 mg for women and a single dose of 5 or 10 mg for men, immediately before bedtime with at least 7-8 hours remaining before the planned time of awakening . Refer to product labeling for detailed information , .
Effects on memory
Controlled studies in adults using objective measures of memory demonstrated no significant evidence of next-day memory impairment after the administration of zolpidem. On the contrary, in a clinical study involving the administration of zolpidem doses of 10 and 20 mg, a marked reduction in a next-morning recall of information relayed to subjects during peak drug effect (90 minutes after dosing) was observed. These subjects experienced a condition known as anterograde amnesia. Subjective evidence from adverse event data has suggested that anterograde amnesia may occur after zolpidem administration, mainly at doses above 10 mg .
Effects on psychomotor function
This drug may cause decreased psychomotor performance. Additive psychomotor effects may occur with other drugs that cause depression of psychomotor function, including alcohol . Patients taking zolpidem should be cautioned against participating in hazardous activities or occupations requiring complete mental alertness or motor coordination, including operating machinery or driving a motor vehicle after ingesting the drug. Potential impairment of the performance of the above types of activities may also occur the day after zolpidem ingestion, especially at higher doses and ingestion of the extended-release form , .
Effects on insomnia and sleep stages
Evidence suggests that this drug is associated with minimal rebound insomnia. During clinical trials with patients using zolpidem on an ‘as-needed’ basis, zolpidem use resulted in global improvements in sleep . Zolpidem has been demonstrated to decrease sleep latency (the time it takes to fall asleep) for up to 35 days in controlled clinical studies . In studies measuring the percentage of sleep time spent in each sleep stage, zolpidem has primarily been shown to preserve sleep stages. Sleep time spent in stages 3 and 4 (deep sleep) was measured as similar to placebo with only minor and inconsistent changes in REM (paradoxical) sleep at the recommended dose .
Next-day residual effects
In 2013, the FDA issued a statement warning that patients who take zolpidem extended-release (Ambien CR)―either 6.25 mg or 12.5 mg―should not drive or participate in other activities requiring full mental alertness the day after taking the drug, due to the fact that zolpidem concentrations can remain increased the next day, and impair the ability to perform these activities , . Patients may decrease their risk of next-morning impairment by taking the lowest dose of their insomnia medicine that treats their symptoms, according to the FDA . Specific dosing recommendations for both men and women are included in this statement . This information is also available on product labeling , .
Rebound effects
There was no polysomnographic (objective) evidence of rebound insomnia at normal doses, in studies evaluating sleep on the nights following discontinuation of zolpidem tartrate. Subjective evidence of impaired sleep in the elderly on the first post-treatment night was observed at doses higher than the recommended 5mg dose for elderly patients .
Metabolism
Zolpidem is metabolized to three pharmacologically by various hepatic cytochrome P450 (CYP) isoenzymes, mainly CYP3A4, but also CYP1A2 and CYP2C9 , .
Although zolpidem is heavily metabolized, all three metabolites are inactive .
The major metabolic routes in humans are oxidation of the methyl group on the phenyl ring or the methyl group on the imidazopyridine moiety, to produce carboxylic acids (metabolites I and II), and hydroxylation of one of the imidazopyridine groups (to produce metabolite X). Another less common pathway is by the oxidation of the methyl groups on the substituted amide .
Properties of Zolpidem
| Melting point: | 189-191°C |
| Density | 1.12±0.1 g/cm3(Predicted) |
| Flash point: | 9℃ |
| storage temp. | Store at RT |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.3 mg/mL |
| form | solid |
| pka | 6.2(at 25℃) |
| color | white |
| Water Solubility | <10mg/L(room temperature) |
| CAS DataBase Reference | 82626-48-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Zolpidem(82626-48-0) |
Safety information for Zolpidem
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Zolpidem
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
