SULFISOMIDINE
Synonym(s):N1-(2,6-Dimethylpyrimidin-4-yl)sulfanilamide;4-Amino-N-(2,6-dimethyl-4-pyrimidinyl)benzenesulfonamide
- CAS NO.:515-64-0
- Empirical Formula: C12H14N4O2S
- Molecular Weight: 278.33
- MDL number: MFCD00010567
- EINECS: 208-204-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
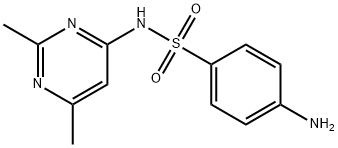
What is SULFISOMIDINE?
Originator
Elkosin,Ciba,US,1951
The Uses of SULFISOMIDINE
Sulfisomidine is a sulfanilamide antibacterial agent.
What are the applications of Application
Sulfisomidin is a sulfonamide small molecule
Definition
ChEBI: A sulfonamide consisting of pyrimidine having methyl substituents at the 2- and 6-positions and a 4-aminobenzenesulfonamido group at the 4-position.
Manufacturing Process
This starting material can be prepared as follows. 123 parts of finely
powdered 6-amino-2,4-dimethylpyrimidine are suspended in 250 parts of dry
pyridine and 222 parts of p-nitrobenzenesulfonyl chloride added at 50°C to
55°C. The whole is then warmed for 2 hours to 55°C. Water is added to the
crystalline aggregate obtained, the precipitated bis-N-(pnitrobenzenesulfonyl)-
6-amino-2,4-dimethylpyrimidine filtered off by suction
and washed with water. It is purified by recrystallizing from methyl ethyl
ketone. On slowly heating it decomposes; on rapidly heating it melts at about
210°C to 215°C with decomposition.
49.3 parts of bis-N-(p-nitrobenzenesulfonyl)-6-amino-2,4-dimethylpyrimidine
are heated to boiling for one hour with 12.3 parts of 6-amino-2,4-
dimethylpyrimidine in 50 parts of dry pyridine. After cooling, the 6-(pnitrobenzenesulfonamido)-
2,4-dimethylpyrimidine formed is precipitated with
water and filtered off by suction. It is purified by dissolving in dilute caustic
soda and precipitating with acid. On recrystallization from dilute alcohol it
melts (with decomposition) at 188°C to 189°C.
On reaction, for example, with iron and hydrochloric acid, 6-(paminobenzenesulfonamido)-
2,4-dimethylpyrimidine, melting point 236°C is
obtained.
brand name
Isosulf;Oestro-gynedron;Poly-gynedron;Sulfamethine;Tricho-gynedron.
Therapeutic Function
Antibacterial
World Health Organization (WHO)
Sulfisomide, a sulfonamide anti-infective agent, was introduced several decades ago for the treatment of bacterial infections. The importance of sulfonamides has subsequently decreased as a result of increasing resistance and their replacement by antibiotics which are generally more active and less toxic. The sulfonamides are known to cause serious adverse effects such as renal toxicity, sometimes fatal exfoliative dermatitis and erythema multiforma and dangerous adverse reactions affecting blood formation such as agranulocytosis and haemolytic or aplastic anaemia. Sulfisomide is still used topically in some countries for vaginal infection.
Pharmaceutical Applications
6-Sulfanilamido-2,4-dimethylpyrimidine (syn: sulphasomidine). A highly soluble sulfonamide with a plasma half-life of 6–8 h. Protein binding is about 90%. Activity is similar to that of sulfadiazine. It is less extensively metabolized than most other sulfonamides and is largely excreted unchanged in the urine.
Properties of SULFISOMIDINE
| Melting point: | 245°C |
| Boiling point: | 294°C (rough estimate) |
| Density | 1.2997 (rough estimate) |
| refractive index | 1.6440 (estimate) |
| storage temp. | 0-6°C |
| solubility | Acetonitrile (Slightly), DMSO (Slightly) |
| pka | pKa 7.25 (Uncertain) |
| form | neat |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 1.382g/L(25 ºC) |
| Merck | 8951 |
| BRN | 261305 |
Safety information for SULFISOMIDINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for SULFISOMIDINE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran








You may like
-
 Sulfisomidine 95% CAS 515-64-0View Details
Sulfisomidine 95% CAS 515-64-0View Details
515-64-0 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2