1-Adamantanamine hydrochloride
Synonym(s):Amantadine hydrochloride;1-Adamantanamine hydrochloride;1-Aminoadamantane hydrochloride;1-Adamantaneammonium chloride;1-Adamantylamine hydrochloride
- CAS NO.:665-66-7
- Empirical Formula: C10H18ClN
- Molecular Weight: 187.71
- MDL number: MFCD00074723
- EINECS: 211-560-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-07 17:19:15
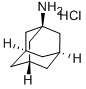
What is 1-Adamantanamine hydrochloride?
Chemical properties
Crystalline Solid
Originator
Symmetrel,DuPont (Endo),US,1966
The Uses of 1-Adamantanamine hydrochloride
1-Adamantanamine hydrochloride is used in the prophylactic or symptomatic treatment of influenza A. It is also used as an antiparkinsonian agent, to treat extra pyramidal reactions, and for postherpetic neuralgia. It is also used an NMDA-receptor antagoinst.
The Uses of 1-Adamantanamine hydrochloride
NMDA-receptor antagonist. Antiviral; antiparkinsonian
The Uses of 1-Adamantanamine hydrochloride
antiviral, antiparkinsonian; treatment of drug-induced extrapyrimidal reactions
The Uses of 1-Adamantanamine hydrochloride
selective FP prostanoid receptor agonist, F-series prostaglandin analog. 200 times as potent as Latanoprost -20oC
What are the applications of Application
Amantadine hydrochloride is an NMDA-receptor antagoinst
Definition
ChEBI: A hydrochloride obtained by combining amantadine and hydrochloric acid in equimolar amounts.
Manufacturing Process
360 ml of 96% sulfuric acid and a solution of 13.6 grams (0.1 mol) of
adamantane in 100 ml of n-hexane were emulsified in the apparatus
described and provided with an inclined centrifugal stirrer. Then a mixture of
46 grams (1.7 mols) of liquid hydrocyanic acid and 29.6 grams (0.4 mol) of
tertiary butanol was added dropwise within 1.5 hours at about 25°C.
After 30 minutes of postreaction, the product was poured on ice. The granular
mass which precipitated [N-(adamantyl-1)formamide] was sucked off and
washed with water. The raw product (37 grams) was then refluxed for 10
hours with a solution of 60 grams of NaOH in 600 ml of diethylene glycol.
After cooling, the solution was diluted with 1.5 liters of water and subjected to
three extractions with ether. The amine was extracted from the ethereal
solution with 2 N HCl and liberated therefrom by the addition of solid NaOH
(while cooling). The alkaline solution was extracted with ether and the
ethereal solution was dried with solid NaOH. Distillation resulted in 10.6 grams
(70% of the theory) of 1-aminoadamantane which, after sublimation, melted
at 180°C to 192°C (seal capillary). It is converted to the hydrochloride.
brand name
Symadine (Solvay Pharmaceuticals); Symmetrel (Endo).
Therapeutic Function
Antiviral, Antiparkinsonian
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biological Activity
amantadine hydrochloride is an antiviral and an antiparkinsonian drug.
Biochem/physiol Actions
Amantadine hydrochloride is effective against influenza viruses both in vivo and in vitro. It is considered as an antagonist of the N-methyl-D-aspartate (NMDA) type glutamate receptor. Amantadine plays an important role in the release of dopamine, preventing dopamine reuptake and blocking microglial activation and neuroinflammation.
Clinical Use
Parkinson’s disease (but not drug-induced
extrapyramidal symptoms)
Post-herpetic neuralgia
Prophylaxis and treatment of influenza A
Safety Profile
Human poison by ingestion. Poison by ingestion, intraperitoneal, and intravenous routes. A human teratogen with developmental abnormalities of the circulatory system. Experimental reproductive effects. Human systemic effects by ingestion: distorted perceptions, euphoria, excitement, hallucinations. When heated to decomposition it emits very toxic fumes of NO, and HCl.
Drug interactions
Potentially hazardous interactions with other drugs
Memantine: increased risk of CNS toxicity - avoid;
effects of amantadine possibly enhanced.
Metabolism
Amantadine is metabolised in the liver to a minor extent, mainly by N-acetylation. The renal amantadine clearance is much higher than the creatinine clearance, suggesting renal tubular secretion in addition to glomerular filtration. After 4-5 days, 90% of the dose appears unchanged in urine. The rate is considerably influenced by urinary pH: a rise in pH brings about a fall in excretion.
storage
Room temperature
Purification Methods
Dissolve the salt in dry EtOH, add a few drops of dry EtOH saturated with HCl gas, followed by dry Et2O to crystallise the hydrochloride. Dry the salt in a vacuum. [Stetter et al. Chem Ber 93 226 1960.]
Properties of 1-Adamantanamine hydrochloride
| Melting point: | >300 °C(lit.) |
| Boiling point: | 308.63°C (rough estimate) |
| Density | 1.0276 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | H2O: 50 mg/mL |
| form | Fine Crystalline Powder |
| color | White |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,374 |
| BRN | 4198854 |
| CAS DataBase Reference | 665-66-7(CAS DataBase Reference) |
| EPA Substance Registry System | Amantadine hydrochloride (665-66-7) |
Safety information for 1-Adamantanamine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for 1-Adamantanamine hydrochloride
| InChIKey | WOLHOYHSEKDWQH-UHFFFAOYSA-N |
New Products
2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Strontium Carbonate, 98% Wang resin Sodium hydrogenphosphate, anhydrous 2-Bromo-3-methoxyaniline hydrochloride, 95% (Custom work) 1-Bromo-4-chlorobenzene, 99% Benzocaine, 98% (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Ramipril Sacubitril- Valsartan Boc-his(trt)-OH Fmoc-L-Glu-OtBu Boc-L-Tyr(tBu)-OH N N Dimethylformamide Dimethyl Acetal (Dmf Dma) Semi carbazide Hydrochloride 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 5-(Difluoromethoxy)-2-Mercaptobenzimidazole Trans-4-Aminocyclohexanol [4tac] 2-Chloromethyl-4-methyl-quinazolineRelated products of tetrahydrofuran
You may like
-
 Amantadine hydrochloride 98%View Details
Amantadine hydrochloride 98%View Details -
 665-66-7 99%View Details
665-66-7 99%View Details
665-66-7 -
 1-Adamantanamine Hydrochloride CAS 665-66-7View Details
1-Adamantanamine Hydrochloride CAS 665-66-7View Details
665-66-7 -
 1-Aminoadamantane hydrochloride CAS 665-66-7View Details
1-Aminoadamantane hydrochloride CAS 665-66-7View Details
665-66-7 -
 Amantadine hydrochloride CAS 665-66-7View Details
Amantadine hydrochloride CAS 665-66-7View Details
665-66-7 -
 15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 -
 6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 -
 Fmoc-Gly-Pro-OH >98%View Details
Fmoc-Gly-Pro-OH >98%View Details
212651-48-4
