Pramoxine hydrochloride
Synonym(s):4-[3-(4-Butoxyphenoxy)propyl]morpholine hydrochloride;Pramoxine hydrochloride
- CAS NO.:637-58-1
- Empirical Formula: C17H28ClNO3
- Molecular Weight: 329.86
- MDL number: MFCD00054323
- EINECS: 211-293-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:53
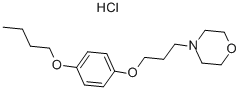
What is Pramoxine hydrochloride?
Description
Pramoxine, also known as pramocaine, is different from other local anesthetic compounds in chemical structure. Active groups which are carboxylic acid derivatives like ester and amide are not present in this molecule. The drug molecule consists of ether functional groups that are much less active on a chemical level. The presence of the free radical morpholine in this compound is the cause of the reduction of the factor of toxicity and pharmacological activity. In pramoxine, toxicity is reduced but the local anesthetic affect is maintained.
Originator
Tronothane, Abbott, US ,1954
The Uses of Pramoxine hydrochloride
Pramoxine Hydrochloride is used as a local anaesthetic and often in anti-itch formulations that provides temporary relief from itching caused by insect bites, minor skin irritations, poison ivy, sunburn and more.
Indications
Pramoxine hydrochloride (Itch-X, PrameGel, Prax, Tronothane, AmLactin AP) is a local anesthetic that is structurally similar to dyclonine and unrelated to the esteror amide-type compounds.Apply to affected area t.i.d. to q.i.d., as directed by a physician. Useful for patients sensitive to ester and/or amide anesthetics. Onset of anesthesia is within minutes. Duration of anesthesia is 2 to 4 hours.
Definition
ChEBI: Pramoxine hydrochloride is an aromatic ether.
Manufacturing Process
About 5.6 g of potassium hydroxide is dissolved in about 150 cc of refluxing ethanol, and then about 16.6 g of hydroquinone monobutyl ether is added to the alcoholic solution. When the hydroquinone is dissolved, about 16.3 g of γmorpholinopropyl chloride (dissolved in a small amount of ethanol) is added to the refluxing solution. The solution is refluxed for about 24 hours and then cooled. The product is recovered by filtering the reaction mixture and then removing the solvent by vacuum distillation. The oily residue is acidified and shaken with ether. The acidic phase is made strongly alkaline with 40% sodium hydroxide, and the oil which separates is extracted into ether. The ethereal phase is dried, and the solvent removed by vacuum distillation. The product distills at 183° to 184°C at a pressure of 2.8 mm. The hydrochloride salt of the foregoing base is prepared by dissolving the base in ether and acidifying with hydrochloric acid and is found to have a MP of 181° to 183°C.
brand name
Tronolane (Ross); Tronothane (Abbott).
Therapeutic Function
Local anesthetic
Clinical Use
Pramoxine is an amino-ether which, like dyclonine, is used as a topical agent in the form of its hydrochloride. Pramoxine hydrochloride may be used for anaesthesia of minor skin wounds and the relief of haemomhoids and other anorectal disorders.The drug is an ingredient of many proprietary topical products, and the U.S.P. has official cream and gel formulations.
Veterinary Drugs and Treatments
A surface and local anesthetic to peripheral nerves not related structurally to procaine-type anesthetics, pramoxine is often added to other topicals to reduce pain and/or itching.
Mode of action
Pramoxine hydrochloride is the hydrochloride salt form of pramoxine, a morpholine derivative with local anesthetic property. Pramocaine hydrochloride decreases the permeability of the neuronal membrane to sodium ions by reversibly binding to and inhibiting voltage-gated sodium channels. This results in stabilization of the membrane and, thereby inhibiting the ionic fluxes required for membrane depolarization, hence resulting in the failure to initiate a propagated action potential and subsequent conduction blockade.
Properties of Pramoxine hydrochloride
| Melting point: | 181-183° |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | neat |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C17H27NO3.ClH/c1-2-3-12-20-16-5-7-17(8-6-16)21-13-4-9-18-10-14-19-15-11-18;/h5-8H,2-4,9-15H2,1H3;1H |
| CAS DataBase Reference | 637-58-1(CAS DataBase Reference) |
Safety information for Pramoxine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pramoxine hydrochloride
| InChIKey | SYCBXBCPLUFJID-UHFFFAOYSA-N |
| SMILES | C1(OCCCN2CCOCC2)=CC=C(OCCCC)C=C1.Cl |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 637-58-1 Pramoxine HCl 98%View Details
637-58-1 Pramoxine HCl 98%View Details
637-58-1 -
 Pramoxine Hydrochloride 637-58-1 99%View Details
Pramoxine Hydrochloride 637-58-1 99%View Details
637-58-1 -
 637-58-1 98%View Details
637-58-1 98%View Details
637-58-1 -
 PRAMOXINE HCL 95-99 %View Details
PRAMOXINE HCL 95-99 %View Details
637-58-1 -
 Pramoxine CAS 637-58-1View Details
Pramoxine CAS 637-58-1View Details
637-58-1 -
 Pramoxine hydrochloride CAS 637-58-1View Details
Pramoxine hydrochloride CAS 637-58-1View Details
637-58-1 -
 Pramoxine hydrochloride CAS 637-58-1View Details
Pramoxine hydrochloride CAS 637-58-1View Details
637-58-1 -
 Pramoxine hydrochloride CAS 637-58-1View Details
Pramoxine hydrochloride CAS 637-58-1View Details
637-58-1
