Flavoxate hydrochloride
Synonym(s):3-Methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylic acid 2-(1-piperidinyl)ethyl ester hydrochloride;DW-61;Rec-7-0040
- CAS NO.:3717-88-2
- Empirical Formula: C24H26ClNO4
- Molecular Weight: 427.92
- MDL number: MFCD00072099
- EINECS: 223-066-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
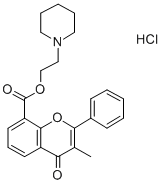
What is Flavoxate hydrochloride?
Chemical properties
Flavoxate hydrochloride is Crystalline Solid
Originator
Urispas,SKF,US,1971
The Uses of Flavoxate hydrochloride
Flavoxate hydrochloride is used as antispasmodic; in treatment of urinary incontinence
The Uses of Flavoxate hydrochloride
Antispasmodic;Phosphodiesterase inhibitor
The Uses of Flavoxate hydrochloride
Smooth muscle relaxant. Used as antispasmodic; in treatment of urinary incontinence.
What are the applications of Application
Flavoxate Hydrochloride is a calcium channel protein inhibitor
Definition
ChEBI: The hydrochloride salt of flavoxate.
Manufacturing Process
A mixture of 13.3 grams of anhydrous aluminum chloride and 100 ml of
carbon disulfide is added to 19.4 grams of 2-propionyloxybenzoic acid
(prepared from the reaction of propionyl chloride and 2-hydroxybenzoic acid).
After an initial evolution of hydrogen chloride, the solvent is removed by
distillation and the mixture is heated at 150° to 160°C for 4 hours. The cooled
reaction mixture is treated with ice and hydrochloric acid and the product, 2-
hydroxy-3-carboxypropiophenone, is obtained from the oily residue by
distillation in vacuo.
A mixture of 1.9 grams of 2-hydroxy-3-carboxypropiophenone, 5.0 grams of
sodium benzoate and 20.0 grams of benzoic anhydride is heated at 180° to
190°C for 6 hours. A solution of 15.0 grams of potassium hydroxide in 50 ml
of ethanol and 20 ml of water is added and refluxed for 1 hour. The mixture is
evaporated and the residue after addition of water yields 3-methylflavone-8-
carboxylic acid.
To a suspension of 12.0 grams of 3-methylflavone-8-carboxylic acid in 200 ml
of anhydrous benzene is added 10.0 grams of thionyl chloride. The mixture is
refluxed for 2 hours during which the suspended solid goes into solution. The
solvent is completely removed by distillation, the residue extracted with
benzene and the extract evaporated to dryness. The product, 3-
methylflavone-8-carboxylic acid chloride, is recrystallized from ligroin to give
crystals melting at 155° to 156°C.
To 11.0 grams of 3-methylflavone-8-carboxylic acid chloride dissolved in 150
ml of anhydrous benzene is added at room temperature 4.8 grams of
piperidinoethanol and the mixture refluxed for 2 to 3 hours. The separated
solid is filtered, washed with benzene and dried. The product, piperidinoethyl
3-methylflavone-8-carboxylate hydrochloride is obtained as a colorless
crystalline solid, MP 232° to 234°C, (from US Patent 2,921,070).
brand name
Urispas (Ortho-McNeil).
Therapeutic Function
Spasmolytic
Side Effects
The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency.
Gastrointestinal: Nausea, vomiting, dry mouth.
CNS: Vertigo, headache, mental confusion, especially in the elderly, drowsiness, nervousness.
Hematologic: Leukopenia (one case which was reversible upon discontinuation of the drug).
Cardiovascular: Tachycardia and palpitation.
Allergic: Urticaria and other dermatoses, eosinophilia and hyperpyrexia.
Ophthalmic: Increased ocular tension, blurred vision, disturbance in eye accommodation.
Renal: Dysuria.
Veterinary Drugs and Treatments
Flovoxate may be considered for treating dogs with detrusor hyperspasticity (hyperactive bladder, urge incontinence).
Pharmacology
Flavoxate hydrochloride counteracts smooth muscle spasm of the urinary tract and exerts its effect directly on the muscle. In a single study of 11 normal male subjects, the time to onset of action was 55 minutes. The peak effect was observed at 112 minutes. 57% of the flavoxate hydrochloride was excreted in the urine within 24 hours.
Properties of Flavoxate hydrochloride
| Melting point: | 232-234°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: ~6.6 mg/mL |
| form | solid |
| color | white |
| InChI | InChI=1S/C24H25NO4.ClH/c1-17-21(26)19-11-8-12-20(23(19)29-22(17)18-9-4-2-5-10-18)24(27)28-16-15-25-13-6-3-7-14-25;/h2,4-5,8-12H,3,6-7,13-16H2,1H3;1H |
| CAS DataBase Reference | 3717-88-2(CAS DataBase Reference) |
Safety information for Flavoxate hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
Computed Descriptors for Flavoxate hydrochloride
| InChIKey | XOEVKNFZUQEERE-UHFFFAOYSA-N |
| SMILES | C12OC(=C(C)C(=O)C=1C=CC=C2C(=O)OCCN1CCCCC1)C1C=CC=CC=1.Cl |
Flavoxate hydrochloride manufacturer
New Products
Trans-methyl 4-aminocyclohexane- carboxylate HCl 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Cyclohexane, (2-propynyloxy)- 3-Bromobenzaldehyde, 95% 2-Naphthol, 98% Cysteamine hydrochloride, 98% Copper(II) bromide, 98% 1-Chloro-2,4-difluorobenzene,98% Dodecylbenzenesulfonic acid, 95% L-Glycine methyl ester.HCl Fmoc-L-Tyr(tBu)-OH Calcium Alphaketoglutarate* H-Ser(t-Bu)-Ser(t-Bu)-Gly-OH Fmoc-Ser(tBu)-Ser(Ψ(Me,Me)pro-OH Triphosgene 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine L-Glutamic Acid Dimethyl Ester Hcl 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base)Related products of tetrahydrofuran
You may like
-
 3717-88-2 Flavoxate hydrochloride 98%View Details
3717-88-2 Flavoxate hydrochloride 98%View Details
3717-88-2 -
 3717-88-2 99%View Details
3717-88-2 99%View Details
3717-88-2 -
 Flavoxate HCl 95.00% CAS 3717-88-2View Details
Flavoxate HCl 95.00% CAS 3717-88-2View Details
3717-88-2 -
 Flavoxate Hydrochloride CAS 3717-88-2View Details
Flavoxate Hydrochloride CAS 3717-88-2View Details
3717-88-2 -
 Flavoxate hydrochloride 3717-88-2 99%View Details
Flavoxate hydrochloride 3717-88-2 99%View Details
3717-88-2 -
 Flavoxate Hydrochloride 3717-88-2 99%View Details
Flavoxate Hydrochloride 3717-88-2 99%View Details
3717-88-2 -
 Flavoxate HCl 99% (HPLC) CAS 3717-88-2View Details
Flavoxate HCl 99% (HPLC) CAS 3717-88-2View Details
3717-88-2 -
 Flavoxate hydrochloride CAS 3717-88-2View Details
Flavoxate hydrochloride CAS 3717-88-2View Details
3717-88-2
