Zirconium tetrachloride
Synonym(s):Tetrachlorozirconium;Zirconium tetrachloride;Zirconium(IV) chloride
- CAS NO.:10026-11-6
- Empirical Formula: Cl4Zr
- Molecular Weight: 233.04
- MDL number: MFCD00011306
- EINECS: 233-058-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
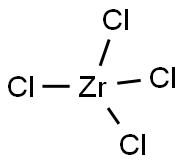
What is Zirconium tetrachloride?
Chemical properties
White, lustrous crystals. Sublimes
above 300C. Soluble in alcohol; decomposes in
water.
Chemical properties
Zirconium tetrachloride forms lustrous, white, monoclinic crystals that melt at 437 ℃. It is a corrosive powder. It is hygroscopic, and thus soluble in cold water, alcohol, ether, and concentrated hydrochloric acid. It reacts vigorously with water forming hydrogen chloride (irritating vapor) and zirconium oxychloride. When moist, zirconium tetrachloride reacts with common materials to form hydrochloric acid that is corrosive to many metals. Through hydrolysis to HCl, zirconium tetrachloride can irritate the respiratory tract and other superficial surfaces of the body on exposure. Zirconium tetrachloride readily forms coordinate bonds with oxygen and nitrogen in organic molecules.
Physical properties
White monoclinic crystals; hygroscopic; density 2.80 g/cm3; sublimes at 331°C; triple point 437°C; vapor pressure 1 torr at 190°C; critical temperature 504.85°C; critical pressure 56.95 atm; critical volume 319 cm3/mol; decomposed by water; soluble in alcohol, ether, and concentrated hydrochloric acid.
The Uses of Zirconium tetrachloride
Zirconium tetrachloride is commonly utilized as a Lewis acid catalyst in many organic synthesis reactions. It is an important source for organometallic complexes of zirconium. It is practiced in many areas like nuclear industry, metal industry, photography, glass, and ceramic industry.
The Uses of Zirconium tetrachloride
For applications in organozirconium chemistry.
1. Activates pyrrolidines for improved conversion, via a modified Bouveault reaction, to the corresponding α,α-dimethylamines.
2. Zirconium (IV) chloride is used as an efficient catalyst in the Pechmann condensation reaction of phenols with β‐keto esters leading to the formation of coumarin derivatives in good yields under solvent‐free conditions.
The Uses of Zirconium tetrachloride
Zirconium(IV) chloride (ZrCl4) is a Lewis acid catalyst, which has low toxicity. It is a moisture resistant material that is used as a catalyst in organic transformations.
ZrCl4 can be used as a catalyst for a variety of organic syntheses, such as Friedel-Crafts reaction, condensation reaction and other reduction reactions.
The Uses of Zirconium tetrachloride
Friedel-Crafts catalyst. Component of Ziegler-type catalysts in the condensation of ethylene. Starting material in the synthesis of a number of organic derivatives of zirconium, such as alkoxides and zircocene. The alkoxides have been shown to be of value in the curing of silicone plastic films. The alkoxyzirconium carboxylates are said to be useful in the water-repellent treatment of textiles and other fibrous materials.
Definition
ChEBI: Zirconium tetrachloride is a zirconium coordination entity comprising four chlorine atoms bound to a central zirconium atom. It has a role as a catalyst. It is a zirconium coordination entity and an inorganic chloride.
Preparation
Zirconium tetrachloride is obtained as an intermediate in recovering zirconium metal from zircon and other minerals (See Zirconium, Recovery). The tetrachloride is obtained by heating a mixture of zirconium hydroxide and car 1004 bon with chlorine gas
Also, tetrachloride can be made by reacting zirconium hydroxide with hydrochloric acid: Zr(OH)4 + 4HCl → ZrCl4 + 4H2O.
General Description
White lustrous crystalline solid. Used as a source of pure zirconium, as a tanning agent, in analytical chemistry and in treating textiles. Zirconium tetrachloride is decomposed by water. Corrosive to metals in the presence of moisture and to tissue.
Air & Water Reactions
Reacts vigorously with water forming hydrochloric acid with the evolution of heat.
Reactivity Profile
Zirconium tetrachloride is corrosive to metals in the presence of moisture and to tissue. Behavior in Fire: Will not burn - sublimes above 626°F (331°C). May give off HCl fumes [USCG, 1999]. Zirconium tetrachloride is incompatible with alcohols (ethanol), lithium metal, and tetrahydrofuran.
Health Hazard
INHALATION: Irritating to upper respiratory tract; presumably caused by liberated HCl. EYES: Irritating. SKIN: Irritating. INGESTION: Burning pain in the mouth and throat, vomiting, watery or bloody diarrhea, retching, collapse, and convulsions.
Fire Hazard
Behavior in Fire: Will not burn - sublimes above 626°F (331°C). May give off HCl fumes.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. Ignites spontaneously in air. When heated to decomposition it emits toxic fumes of Cl-. See also ZIRCONIUM COMPOUNDS and HYDROCHLORIC ACID.
Purification Methods
Crystallise it repeatedly from conc HCl. It is hydrolysed by H2O to form white ZrOCl2 (see below) and HCl. [Krebs Z Anorg Allgem Chem 378 263 1970.]
Properties of Zirconium tetrachloride
| Melting point: | 437 °C |
| Boiling point: | 331°C |
| Density | 2.8 g/mL at 25 °C(lit.) |
| vapor pressure | 1 mm Hg ( 190 °C) |
| storage temp. | Store below +30°C. |
| solubility | Soluble in alcohol, ether, hydrochloric acid. |
| form | powder |
| color | White |
| Specific Gravity | 2.803 |
| PH | <1 (H2O, 20℃)(saturated aqueous solution) |
| Water Solubility | Reacts with water. |
| Sensitive | Moisture Sensitive & Hygroscopic |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Merck | 14,10174 |
| Exposure limits | ACGIH: TWA 5 mg/m3; STEL 10 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 5 mg/m3; STEL 10 mg/m3 |
| CAS DataBase Reference | 10026-11-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Zirconium tetrachloride(10026-11-6) |
| EPA Substance Registry System | Zirconium tetrachloride (10026-11-6) |
Safety information for Zirconium tetrachloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zirconium tetrachloride
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Zirconium tetrachloride 98%View Details
Zirconium tetrachloride 98%View Details -
 Zirconium(IV) chloride, 99.5% CAS 10026-11-6View Details
Zirconium(IV) chloride, 99.5% CAS 10026-11-6View Details
10026-11-6 -
 Zirconium(IV) chloride, anhydrous, powder trace metals basis CAS 10026-11-6View Details
Zirconium(IV) chloride, anhydrous, powder trace metals basis CAS 10026-11-6View Details
10026-11-6 -
 Zirconium(IV) chloride CAS 10026-11-6View Details
Zirconium(IV) chloride CAS 10026-11-6View Details
10026-11-6 -
 Zirconium(IV) chloride CAS 10026-11-6View Details
Zirconium(IV) chloride CAS 10026-11-6View Details
10026-11-6 -
 Zirconium(IV) chloride, 98% CAS 10026-11-6View Details
Zirconium(IV) chloride, 98% CAS 10026-11-6View Details
10026-11-6 -
 Zirconium(iv) chloride 95% CAS 10026-11-6View Details
Zirconium(iv) chloride 95% CAS 10026-11-6View Details
10026-11-6 -
 Zirconium (iv) ChlorideView Details
Zirconium (iv) ChlorideView Details
10026-11-6
