Titanium tetrachloride
Synonym(s):Titanium tetrachloride;Titanium(IV) chloride;TTC
- CAS NO.:7550-45-0
- Empirical Formula: Cl4Ti
- Molecular Weight: 189.68
- MDL number: MFCD00011267
- EINECS: 231-441-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
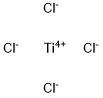
What is Titanium tetrachloride ?
Description
Titanium ore was first discovered in 1791 in Cornish beach
sands by an English clergyman, William Gregor. The actual
identification of the oxide was made a few years later by
a German chemist, M.H. Klaproth, who gave the metal
constituent of this oxide the name titanium, after the Titans of
Greek mythology. Pure metallic titanium was first produced in
the early 1900s in 1910 by M.A. Hunter at Rensselaer Polytechnic
Institute in cooperation with General Electric
Company.
Titanium tetrachloride is an inorganic compound that is an
important intermediate in the production of titanium metal
and the pigment titanium dioxide. On contact with humid air,
it forms opaque clouds of titanium dioxide (TiO2) and
hydrogen chloride (HCl). Early attempts to isolate titanium
metal from titanium tetrachloride were unsuccessful. The
process was improved and commercialized by William Kroll of
Luxembourg in the 1930s which involved the reduction of
titanium tetrachloride with magnesium in an inert gas atmosphere.
This process remains essentially unchanged today. The
primary use of titanium tetrachloride is for titanium dioxide
used in paints.
The production of titanium metal accounts for only 5% of
annual titanium mineral consumption, with the remainder
being used in the titanium pigment industry. Pigments are
produced using either a sulfate process or a more environmentally
acceptable carbochlorination process that converts
TiO2 into TiCl4. The latter process also supplies the TiCl4
necessary for the production of titanium metal.
Chemical properties
Colorless liquid. Fumes strongly when exposed to moist air, forming a dense and persistent white cloud. Soluble in dilute hydrochloric acid; soluble in water with evolution of heat; concentrated aqueous solutions are stable and corrosive; dilute solutions precipitate insoluble basic chlorides.
Chemical properties
Description: Titanium tetrachloride is a noncombustible, colorless to light yellow liquid that fumes in air. Penetrating acrid odor.
The Uses of Titanium tetrachloride
Titanium tetrachloride is used as an intermediate in the
manufacture of titanium metal, titanium dioxide, titanous
chloride pigments, iridescent glass, and artificial pearls and as
a starting material for a variety of organic and inorganic titanium
compounds. It is also used as a dye, a polymerization
catalyst, and as a catalyst in many organic syntheses because of it acidity and oxophilicity in many applications in the chemical
industry. Titanium tetrachloride was formerly used as a smokeproducing
screen with ammonia for the military; however, due
to its extremely irritating and corrosive qualities in both liquid
and smoke formulation, military applications are rarely used.
The conversion of tetrachloride to titanium metal takes
place by the reduction of chloride with magnesium which
yields titanium metal and magnesium chloride and is referred
to as the Kroll process after its inventor:
2 Mg + TiCl4→2 MgCl2 + Ti
The Uses of Titanium tetrachloride
Activates pyrrolidines for improved conversion, via a modified Bouveault reaction, to the corresponding α,α-dimethylamines.1
The Uses of Titanium tetrachloride
manufacture of titanium compounds, iridescent glass and artificial pearls. Formerly used with potassium bitartrate as a mordant in textile industry, and with dyewoods in dyeing leather; also as smoke-producing screen with ammonia.
The Uses of Titanium tetrachloride
Titanium (IV) tetrachloride (TiCl4) produces a dense white smoke-like vapor when exposed to moist air. It is used as smoke screens and for skywriting, as well in theatrical productions where fog or smoke is required for the scene.
Production Methods
TiCl4 is used in TiO2 production, the manufacture of artificial pearls and iridescent glass, and, by the military, to create smoke screens.
General Description
A colorless fuming liquid with a pungent odor. Corrosive to metals and tissue. Very toxic by inhalation.
Reactivity Profile
Titanium tetrachloride acts as an acid in aqueous solution. During the reduction of Titanium tetrachloride to titanium metal with potassium, an explosion occurred. The system had been heated to 90°C [Walter and Mandell 1967]. Addition directly to tetrahydrofuran caused a violent exothermic reaction [Inorg. Syn., 1982, 21, 135]. Ethylene can polymerize at low pressure if catalyzed by titanium halides. (Sundaram, K. M, M. M. Shreehan, E. F. Olszewski. thylene. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2001.)
Hazard
Toxic by inhalation, strong irritant to skin and tissue.
Health Hazard
Titanium tetrachloride is a highly corrosive, acute irritant to the skin, eyes, mucous membranes and the respiratory tract. It is capable of causing death or permanent injury due to exposures encountered in normal use. Even short contact may lead to eye inflammation which may result in corneal opacities.
Fire Hazard
Material will react with water to produce hydrochloric acid. Titanium tetrachloride may ignite other combustible materials (e.g., wood, oil, etc.). Flammable, poisonous gases may accumulate in tanks and hopper cars. Runoff to sewer may create fire or explosion hazard. Reacts strongly with water to release hydrochloric acid and heat. Avoid water, moist air. Stable in concentrated aqueous solutions. Avoid contact with moisture; the chemical absorbs moisture from air and evolves dense white fumes.
Safety Profile
Poison by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Cl-. See also TITANIUM COMPOUNDS.
Potential Exposure
Used in the manufacture of titanium salts; mordant dye; titanium pigments; and used as a chemical intermediate for titanium metal; titanium dioxide; as an agent in smoke screens; polymerization catalyst; and iridescent agent in glass and pearl manufacturing.
Carcinogenicity
Rats exposed to 10 mg TiCl4/m3 for 6 h/day, 5 days/week, for 2 years developed rhinitis, tracheitis, hyperplasia, foamy dust cell accumulation, and alveolar bronchiolization. In addition, 5/150 animals developed squamous cell carcinoma, compared to 0/156 in the controls. Two of the squamous cell carcinomas were described as cystic keratinizing lesions, whose relevance to humans was questioned by the authors. However, the remaining three squamous cell tumors were described as microscopic, well-differentiated carcinomas. Therefore, TiCl4 may be regarded as potentially carcinogenic in the rat.
Environmental Fate
Titanium tetrachloride is a colorless to pale yellow liquid with
a strong penetrating odor. If it comes in contact with water, it
rapidly forms hydrochloric acid, as well as titanium
compounds. Titanium tetrachloride is not found naturally in
the environment and is made from minerals that contain titanium.
Titanium tetrachloride is the most toxic of the titanium
compounds and is highly corrosive and unstable and
undergoes rapid hydrolysis through a vigorous exothermic
reaction generating a large quantity of heat and hydrolysis
products such as hydrochloric acid and other titanium
compounds including titanium hydroxide, titanium oxychloride,
and titanium dioxide. It may ignite other combustible
materials (e.g., wood, oil, etc.) and produce toxic gases. Runoff
to sewers may create fires or explosion hazards.
Titanium tetrachloride enters the environment primarily as
air emissions from facilities that make or use it in these various
chemical processes or as a result of accidental releases. Its
chemical properties suggest that titanium tetrachloride partitions
to the air underscoring the fact that the most likely route
of human exposure to titanium tetrachloride hydrolysis or its
intermediate products is via inhalation. The hydrochloric acid
may break down or be carried in the air for some distance.
Some of the titanium compounds may settle out to soil or in
water as bottom sediments and remain for many years. Other
titanium compounds, such as titanium dioxide, can also be
found in the air. Consequently, environmental transport of
titanium tetrachloride is negligible in soil and water; however,
the atmospheric transport of the hydrolysis products may be
significant. There is a paucity of data estimating the residence
time for titanium tetrachloride in air or water; however, based
on the compounds’ rapid hydrolysis, residence times are
expected to be in the order of hours. In water, hydrochloric
acid dissociates to the hydrogen and chloride ions, while
titanium dioxide is insoluble in water and may settle out into
the sediments. Titanium tetrachloride released to soils or
sediments is expected to hydrolyze on contact with moisture in
the soil and sediment. However, titanium dioxide is likely to
remain in the soil or settle out to the sediment as it is an inert
compound.
The chemical characteristics of titanium tetrachloride and its
rapid hydrolysis in the presence of water suggest that there is
little potential for bioaccumulation or biomagnification in
aquatic or terrestrial organisms. It is not possible to determine
if there is a potential for bioaccumulation of the compound in
humans. Because of its chemical characteristics and rapid
hydrolysis in the presence of water, however, it is also unlikely
that it would bioaccumulate in the body although its final
hydrolysis product may do so. Hence, titanium tetrachloride is
not considered to be persistent, bioaccumulating, nor toxic.
Shipping
UN1838 Titanium tetrachloride, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 8-Corrosive material, Inhalation Hazard Zone B.
Purification Methods
Reflux it with mercury or a small amount of pure copper turnings to remove the last traces of colour [due to FeCl3 and VCl4], then distil it under N2 in an all-glass system, taking precautions to exclude moisture. Clabaugh et al. [J Res Nat Bur Stand 55 261 1955] removed organic material by adding aluminium chloride hexahydrate as a slurry with an equal amount of water (the slurry being ca one-fiftieth the weight of TiCl4), refluxed it for 2-6hours while bubbling in chlorine, the excess of which is subsequently removed by passing a stream of clean dry air. The TiCl4 is then distilled, refluxed with copper and again distilled, taking precautions to exclude moisture. Volatile impurities are then removed using a technique of freezing, pumping and melting. The titanium tetrachloride 2-tetrahydrofuran complex [Beilstein 17/1 V 33.] M 333.9, has m 126-128o and is easier to handle than TiCl4 [Abrahamson et al. Organometallics 3 1379 1984]. [Baxter & Fertig J Am Chem Soc 45 1228 1923, Baxter & Butler J Am Chem Soc 48 3117 1926.] HARMFUL VAPOURS.
Toxicity evaluation
It has been hypothesized that harmful effects of titanium
tetrachloride are due to the vigorous reaction with water from
perspiration on the skin, tears, and moisture in the air resulting
in a severely exothermic reaction. The mechanism of injury is
thought to be a combined thermal and acid burn process.
Initially, there is a thermal burn, which exposes deeper tissue
layers to hydrolysis products such as hydrochloric acid,
furthering the severity of the effects.
The results of a mouse study showed that titanium
tetrachloride was more toxic than hydrochloric acid. It is
speculated that the more severe effects seen from exposure
to titanium tetrachloride compared with hydrochloric acid
is because hydrochloric acid is dissolved in the moisture of
the nasopharynx and trachea remaining in this upper
respiratory area and therefore is physically limited in the
extent of lung penetration. However, in the case of exposure
to titanium tetrachloride, the hydrolysis occurs in several
steps. One of the hydrolysis products, titanium oxide
hydrate, is a particulate that can adsorb the hydrochloric
acid vapors that are also generated during hydrolysis and
carry them into the deeper parts of the lungs and to the
alveoli. However, titanium tetrachloride hydrolysis products
such as titanium oxide hydrate can absorb some of the
hydrochloric acid vapors that are also generated during
hydrolysis and carry them past the upper respiratory spaces
and into the deeper parts of the lungs. This mechanism of
toxicity could potentially explain the second- and thirddegree
burns observed after acute dermal exposure to titanium
tetrachloride.
Incompatibilities
Violent reaction with water or steam, releasing heat and hydrogen chloride fumes. Contact with moist air releases hydrogen chloride. Attacks many metals in presence of moisture.
Properties of Titanium tetrachloride
| Melting point: | −25 °C(lit.) |
| Boiling point: | 135-136 °C(lit.) |
| Density | 1.73 g/mL at 20 °C(lit.) |
| vapor pressure | 50 mm Hg ( 55 °C) |
| refractive index | 1.61 |
| Flash point: | 46 °F |
| storage temp. | Flammables area |
| solubility | H2O: soluble |
| form | Solution |
| appearance | Colorless liquid |
| color | Light yellow to dark brown |
| Specific Gravity | 1.726 |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,9478 |
| Exposure limits | ACGIH: TWA 50 ppm OSHA: TWA 25 ppm; STEL 125 ppm NIOSH: IDLH 2300 ppm |
| Dielectric constant | 40.0(Ambient) |
| Stability: | Stable. Reacts with water. Incompatible with moisture, ammonia, amines, alcohols, potassium and other chemically active metals. |
| CAS DataBase Reference | 7550-45-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Titanium tetrachloride(7550-45-0) |
| EPA Substance Registry System | Titanium tetrachloride (7550-45-0) |
Safety information for Titanium tetrachloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Titanium tetrachloride
Titanium tetrachloride manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Titanium Tetrachloride 1M in Dichloromethane CAS 7550-45-0View Details
Titanium Tetrachloride 1M in Dichloromethane CAS 7550-45-0View Details
7550-45-0 -
 Titanium tetrachloride solution 1.0 M in toluene CAS 7550-45-0View Details
Titanium tetrachloride solution 1.0 M in toluene CAS 7550-45-0View Details
7550-45-0 -
 Titanium tetrachloride CAS 7550-45-0View Details
Titanium tetrachloride CAS 7550-45-0View Details
7550-45-0 -
 Titanium tetrachloride, 99.9% CAS 7550-45-0View Details
Titanium tetrachloride, 99.9% CAS 7550-45-0View Details
7550-45-0 -
 Titanium(IV) Chloride (14% in Dichloromethane, ca. 1.0mol/L) CAS 7550-45-0View Details
Titanium(IV) Chloride (14% in Dichloromethane, ca. 1.0mol/L) CAS 7550-45-0View Details
7550-45-0 -
 Titanium Tetrachloride 1M in Toluene CAS 7550-45-0View Details
Titanium Tetrachloride 1M in Toluene CAS 7550-45-0View Details
7550-45-0 -
 Titanium tetrachloride solution 1.0 M in methylene chloride CAS 7550-45-0View Details
Titanium tetrachloride solution 1.0 M in methylene chloride CAS 7550-45-0View Details
7550-45-0 -
 Titanium (IV) tetrachloride 99% CAS 7550-45-0View Details
Titanium (IV) tetrachloride 99% CAS 7550-45-0View Details
7550-45-0
