Zinc bromide
Synonym(s):Zinc bromide;Zinc dibromide
- CAS NO.:7699-45-8
- Empirical Formula: Br2Zn
- Molecular Weight: 225.2
- MDL number: MFCD00011294
- EINECS: 231-718-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
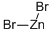
What is Zinc bromide?
Description
Zinc bromide is a Lewis acid. Zinc bromide is sometimes used for the selective removal of Boc protecting groups from secondary amines.
Chemical properties
Zinc bromide is an odorless white crystalline solid or white hygroscopic powder.
Physical properties
White crystalline powder; sharp metallic taste; orthorhombic structure; refractive index 1.5452; density 4.20 g/cm3; very hygroscopic; melts at 394°C; vaporizes at 650°C; highly soluble in water 447g/100 mL at 20°C; aqueous solution acidic; very soluble in alcohol, ether, and acetone; soluble in alkali hydroxides and ammonia solution.
The Uses of Zinc bromide
Zinc bromide is a white crystalline powder prepared by dissolving zinc carbonate in hydrobromic acid.
Zinc chloride (ZnCl2) is a white granular crystal made by the action of hydrochloric acid on zinc.
Zinc iodide (ZnI2) is a white powder made by dissolving zinc in ionic acid. All of the zinc halides are soluble in water, alcohol, and ether. They were all used as halides for the collodion emulsion processes.
The Uses of Zinc bromide

To a solution the SM (0.34 g, 0.76 mmol) in DCM (10 mL) was added ZnBr2 (0.70 g, 3.1 mmol). The reaction mixture was stirred at RT for 3 days, after which time it was diluted with aq Na2CO3 and extracted with DCM. The combined organics were washed with brine, dried (Na2SO4), and concentrated to provide the product as a clear film. [0.25 g]
The Uses of Zinc bromide
Optimal catalyst for stereospecific and regioselective reaction of silacyclopropanes with carbonyl compounds.1
The Uses of Zinc bromide
Making silver bromide collodion emulsions for photography; in the shielding of viewing windows for nuclear reactions.
What are the applications of Application
Zinc bromide is a compound used as a lewis acid in organic chemistry
Preparation
Zinc bromide is prepared by mixing barium bromide and zinc sulfate solutions. The product barium sulfate is removed by filtration and the filtrate is evaporated to obtain crystals of zinc bromide: BaBr2 + ZnSO4 → ZnBr2 + BaSO4
Zinc bromide also may be prepared by the action of zinc with hydrobromic acid followed by crystallization.
General Description
A white crystalline noncombustible solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Zinc bromide is used in medicine, in photography.
Air & Water Reactions
Hygroscopic. Water soluble
Reactivity Profile
Acidic inorganic salts, such as Zinc bromide, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Inhalation of dust may irritate nose and throat. Ingestion can cause irritation or corrosion of the alimentary tract; if large amount is swallowed and not thrown up, drowsiness and other symptoms of bromide poisoning may occur. Contact with eyes or skin causes irritation.
Flammability and Explosibility
Non flammable
Potential Exposure
Zinc bromide is used in photography, rayon manufacturing and medicine
Shipping
UN3260 Corrosive solid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Heat ZnBr2 to 300o under vacuum (2x10-2 mm) for 1hour, then sublime it. [Wagenknecht & Juza Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1072 1965.]
Incompatibilities
Keep away from alkali metals. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, metallic sodium, or potassium. Store above 32 ℉/0℃.
Properties of Zinc bromide
| Melting point: | 394 °C (lit.) |
| Boiling point: | ~670 °C/1 atm (lit.) |
| Density | 4.22 |
| refractive index | 1.5452 |
| Flash point: | 650°C |
| storage temp. | Store below +30°C. |
| solubility | Soluble in alcohol, ether, acetone and tetrahydrofuran. |
| appearance | White crystalline powder |
| form | beads |
| color | White to pale cream |
| Specific Gravity | 4.2012 |
| PH | 4 (H2O, 20℃)(saturated solution) |
| Water Solubility | soluble, 447 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,1029 |
| Stability: | Stable. Forms explosive mixtures with sodium and potassium. Protect from moisture. |
| CAS DataBase Reference | 7699-45-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Zinc dibromide(7699-45-8) |
| EPA Substance Registry System | Zinc bromide (7699-45-8) |
Safety information for Zinc bromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zinc bromide
Zinc bromide manufacturer
JSK Chemicals
Sainor Laboratories Pvt Ltd Unit III
Axiom Chemicals Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Zinc bromide, Ultra dry CAS 7699-45-8View Details
Zinc bromide, Ultra dry CAS 7699-45-8View Details
7699-45-8 -
 Zinc bromide, Ultra dry CAS 7699-45-8View Details
Zinc bromide, Ultra dry CAS 7699-45-8View Details
7699-45-8 -
 Zinc bromide hydrate CAS 7699-45-8View Details
Zinc bromide hydrate CAS 7699-45-8View Details
7699-45-8 -
 Zinc bromide hydrate CAS 7699-45-8View Details
Zinc bromide hydrate CAS 7699-45-8View Details
7699-45-8 -
 Zinc bromide CAS 7699-45-8View Details
Zinc bromide CAS 7699-45-8View Details
7699-45-8 -
 Zinc bromide CAS 7699-45-8View Details
Zinc bromide CAS 7699-45-8View Details
7699-45-8 -
 Zinc bromide CAS 7699-45-8View Details
Zinc bromide CAS 7699-45-8View Details
7699-45-8 -
 Zinc bromide 1.9M in 2-Methyl THF 99%View Details
Zinc bromide 1.9M in 2-Methyl THF 99%View Details
