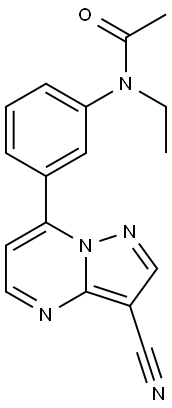
Zaleplon
Synonym(s):CL-284846;N-[3-(3-Cyanopyrazolo-[1,5-a]pyrimidin-7-yl)phenyl)-N-ethylacetamide
- CAS NO.:151319-34-5
- Empirical Formula: C17H15N5O
- Molecular Weight: 305.33
- MDL number: MFCD00867990
- EINECS: 604-794-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:47
What is Zaleplon?
Absorption
Absorption Zaleplon is rapidly and almost completely absorbed following oral administration.
Toxicity
Side effects include abdominal pain, amnesia, dizziness, drowsiness, eye pain, headache, memory loss, menstrual pain, nausea, sleepiness, tingling, weakness
Description
Zaleplon was introduced in Sweden and Denmark as a new treatment for insomnia, particularly in patients who have difficulty in falling asleep. Zaleplon is a non-benzodiazepine compound and is the first in a new generation belonging to the pyrazolopyrimidine class, showing therefore fewer benzodiazepine-like side effects. It can be synthesized in 3 steps from the corresponding acetophenone, the key step being the cyclization of the appropriate enaminone with 3-aminopyrazole-4-carbonitrile. Biochemically, Zaleplon is a full agonist at the benzodiazepine o)1 site of the gaba-A receptor complex, but its behavioural profile remains distinct from both benzodiazepine (e.g. Lorazepam) or non-benzodiazepine (e.g. Zopiclone or Zolpidem) sedativehypnotic drugs. Clinical pharmacokinetic analysis showed rapid absorption and elimination. In man, the main metabolic route was oxidative giving the major metabolites 5-oxo Zaleplon and its N-desethyl analog. Both were shown to have no effect at central benzodiazepine receptors and to be rapidly excreted as glucuronides. In patients with chronic insomnia, Zaleplon at 5 and 10 mg/kg significantly reduced sleep latency and improved the quality of sleep compared with placebo without altering the normal sleep architecture. Given its short halflife, the next-day residual effects such as hangover are minimized. It may have some advantages over benzodiazepines regarding unwanted amnesic effects and psychomotor impairment. There was no evidence for the occurrence of rebound insomnia at 10 mg/kg.
Description
Zaleplon (Item No. 11577) is an analytical reference material that is functionally categorized as a sedative. It is a pyrazolopyrimidine that selectively activates the GABAA α1 receptor subunit (EC50 = 1.1 μM), producing a sedative effect. It is characterized as being an ultra-short-acting sedative due to short Tmax (0.7-1.4 hours) and half-life (1 hour) values. This product is intended for research and forensic applications.
Chemical properties
Off-White Powder
Originator
American Home Products (US)
The Uses of Zaleplon
Selective non-benzodiazepine GABAA receptor agonist
The Uses of Zaleplon
topical antibacterial (topical)
Background
Zaleplon is a sedative/hypnotic, mainly used for insomnia. It is known as a nonbenzodiazepine hypnotic. Zaleplon interacts with the GABA receptor complex and shares some of the pharmacological properties of the benzodiazepines. Zaleplon is a schedule IV drug in the United States.
Indications
For the treatment of short-term treatment of insomnia in adults.
Definition
ChEBI: A pyrazolo[1,5-a]pyrimidine having a nitrile group at position 3 and a 3-(N-ethylacetamido)phenyl substituent at the 7-position.
Manufacturing Process
N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl]acetamide amide
A 1 gram-equivalent portion of N-(3-acetylphenyl)ethanamide in equivalent
portion of dimethylformamide dimethyl acetal was refluxed for 8 hours, then
evaporated. The residue was taken up in 200 ml of dichloromethane, passed
through hydrous magnesium silicate, diluted with hexane and concentrated,
giving the desired compound.
N-[3-[3-(Dimethylamino)-1-oxo-2-propenyl]phenyl-N-ethylacetamide
A mixture of 1 gram-equivalent of N-[3-[3-(dimethylamino)-oxo-2-
propenyl]phenyl]propanamide and equivalent portion of 60% sodium hydride
in oil in dimethylformamide was stirred for 0.5 hour under argon, then cooled
in an ice bath and a solution of 1gram-equivalent of ethyl iodide in 10 ml of
dimethylformamide was added in small portions. The mixture was then stirred
at room temperature for 0.5 hour and extracted three times with hexane. The
extracts were discarded, water was added and this mixture extracted with
dichloromethane. This extract was evaporated and the residue crystallized
from hexane giving the desired compound, MP 110°-113°C.
N-[3-(3-Cyanopyrazolo[1,5-a]pyrimidin-7-yl)phenyl]-N-ethylacetamide
A mixture of 1 gram-equivalent of 3-aminopyrazole-4-carbonitrile and 1 gram-
equivalent of N-[3-[3-(dimethylamino)-1-oxo-2-propenyl]phenyl]-N-
ethylacetanamide in 50 ml of glacial acetic acid was refluxed for 8 hours and
then the solvent was removed. The residue was partitioned between saturated
aqueous sodium bicarbonate and dichloromethane. The organic layer was
separated, dried, passed through a pad of hydrous magnesium silicate and
hexane was added to the refluxing filtrate. The mixture was then cooled and
the solid collected, giving the desired product, MP 186°-187°C.
brand name
Sonata (Jones).
Therapeutic Function
Sedative
General Description
Zaleplon (Sonata, a pyrazolopyrimidine) isanother short-acting nonbenzodiazepine hypnotic.Pharmacologically and pharmacokinetically, zaleplon is similarto zolpidem; both are hypnotic agents with short halflives.It also has selective high affinity for α1-subunit containingBzRs but produces effects at other BzR/GABAAsubtypes as well. Zaleplon is well absorbed following oraladministration with an absolute bioavailability of approximately30% because of significant presystemic metabolism.It exhibits a mean half-life of approximately 1 hour, with lessthan 1% of the dose excreted unchanged in urine. It is primarilymetabolized by aldehyde oxidase to 5-oxo-zaleplon andis also metabolized to a lesser extent by CYP3A4. Ndemethylationyields desethylzaleplon, which is quickly converted,presumably by aldehyde oxidase, to 5-oxo-desethylzaleplon.These oxidative metabolites are thenconverted to glucuronides and eliminated in urine. All of zaleplon’smetabolites are pharmacologically inactive. It mayhave a more rapid onset (about 1 hour) and termination of actionthan zolpidem, and therefore, it is good to initiate sleepinstead of keeping sleep.
Biological Activity
Non-benzodiazepine agent that acts as an agonist at the benzodiazepine site. Displays hypnotic, anxiolytic, myorelaxant and anticonvulsant activity.
Pharmacokinetics
Zaleplon is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class and is indicated for the short-term treatment of insomnia. While Zaleplon is a hypnotic agent with a chemical structure unrelated to benzodiazepines, barbiturates, or other drugs with known hypnotic properties, it interacts with the gamma-aminobutyric acid-benzodiazepine (GABABZ) receptor complex. Subunit modulation of the GABABZ receptor chloride channel macromolecular complex is hypothesized to be responsible for some of the pharmacological properties of benzodiazepines, which include sedative, anxiolytic, muscle relaxant, and anticonvulsive effects in animal models. Zaleplon also binds selectively to the CNS GABAA-receptor chloride ionophore complex at benzodiazepine(BZ) omega-1 (BZ1, ο1) receptors.
Pharmacokinetics
Zaleplon displays a unique binding profile with GABAA that is distinct from the benzodiazepines but similar to that of zolpidem. Because of it greater potency for GABAA, the starting dose for zaleplon is comparable to that of zolpidem. It is rapidly absorbed, with a log P of 1.23, although only 30% of the dose is bioavailable because of rapid first-pass metabolism via liver cytosolic aldehyde oxidase/xanthine oxidase (molybdenum hydroxylases) to its major ring oxidation product, 5-oxo-zaleplon metabolite. The minor metabolism pathways include N-dealkylation from microsomal oxidation via CYP3A4 to N-desethyl-zaleplon and N-desethyl-5-oxo-zaleplon. It is rapidly metabolized by the liver, with an elimination half-life of approximately 1 hour. The oxidative metabolites are inactive, conjugated with glucuronic acid, and eliminated in the urine. Inhibitors of CYP3A4 and aldehyde oxidase can increase the plasma concentration of zaleplon significantly, although this usually does not require dosage modification. Zaleplon does not accumulate with once-daily administration and displays linear pharmacokinetics in the therapeutic range.
Metabolism
Zaleplon is primarily metabolized by aldehyde oxidase.
Metabolism
The elimination half-life of zaleplon is increased in patients with hepatic insufficiency, requiring an adjustment in dosage. High-fat meals increase the time to peak concentration and decrease the plasma concentration without affecting the half-life. These results suggest that for faster sleep onset, zaleplon should not be administered either with or immediately after a meal, which increases the time to reach peak plasma concentrations. In short-term studies (2–5 weeks), zaleplon has been shown to improve sleep quality with minimal adverse effects and no significant rebound insomnia on stopping the drug. Because of its short elimination half-life, zaleplon is quite good at getting people to sleep but is not as good at keeping people asleep. Unlike with zolpidem and eszopiclone, it has been proposed that if the patient awakens in the middle of the night (with ≥4 hours of sleep time remaining), another dose of zaleplon can be taken.
Properties of Zaleplon
| Melting point: | 186-1870C |
| Density | 1.25±0.1 g/cm3(Predicted) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: ~20mg/mL |
| pka | -1.47±0.50(Predicted) |
| form | Solid |
| color | white |
| CAS DataBase Reference | 151319-34-5(CAS DataBase Reference) |
Safety information for Zaleplon
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Zaleplon
Zaleplon manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1