Eszopiclone
- CAS NO.:138729-47-2
- Empirical Formula: C17H17ClN6O3
- Molecular Weight: 388.81
- MDL number: MFCD03700720
- EINECS: 202-303-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
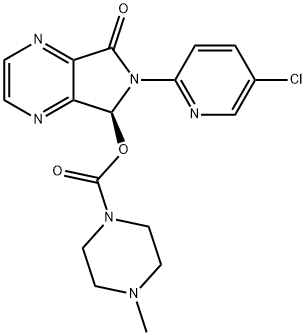
What is Eszopiclone?
Absorption
Eszopiclone is rapidly absorbed and the peak concentration is reached within about 1 hour after oral administration. The mean AUC after a 3 mg dose of eszopiclone was 278 ng/mL × h. The consumption of a high-fat has been shown to slow absorption. Steady-state concentrations of eszopiclone are reached within 24-48 hours.
Toxicity
The oral LD50 of eszopiclone in rats is 980 mg/kg and 3200 mg/kg in rabbits. Symptoms of overdose may include mental status changes and somnolence, demonstrating general exaggeration of the drug's pharmacological effects. Perform gastric lavage and offer supportive treatment if an overdose is suspected, including intravenous fluids as required. Flumazenil may be used. Vital signs should be closely monitored in addition to patient symptoms. Appropriate medical interventions should be employed. The possibility of an overdose with multiple drugs should be considered. Ensure to contact the local poison control center for the most updated management of hypnotic drug overdose.
Description
Eszopiclone is a non-benzodiazepine hypnotic agent indicated for the treatment of insomnia to induce sleep and for sleep maintenance. It has similar pharmacokinetic and pharmacodynamic parameters as the previously marketed non-benzodiazepine hypnotics zolpidem and zaleplon. However, unlike its predecessors, eszopiclone is not restricted to short-term treatment of insomnia. Clinical studies of up to 6 months of use show that patients do not develop tolerance to its effect. Eszopiclone is the (S)-enantiomer of zopiclone, which has been marketed as the racemic mixture in Europe for almost 20 years. These agents belong to the cyclopyrrolone class of drugs that act as agonists at the type A GABA receptor. Eszopiclone has approximately 50-fold higher binding affinity than its antipode (R)-zopiclone for GABA-A receptor (IC50=21 and 1130 nM, respectively). In addition, the two enantiomers exhibit significant differences in their pharmacokinetic parameters and in vivo efficacy. In healthy volunteers, eszopiclone has 2-fold higher Cmax and 2-fold greater elimination half-life than the (R)-enantiomer.The two most frequent adverse events associated with eszopiclone treatment are unpleasant taste and headache. Other less frequent side effects include somnolence, dry mouth, and nausea.
Chemical properties
White To Pale Yellow
Originator
Aventis (France)
The Uses of Eszopiclone
Eszopiclone is the active stereoizomer of Zopiclone and belongs to the class of drug known as cyclopyrrones. It is a nonbenzodiazepine hypnotic agent used as a treatment for insomnia. This is a controlled substance (depressant) in the US but not in Canada.
Indications
Eszopiclone is indicated for the treatment of insomnia.
Background
Eszopiclone, marketed by Sepracor under the brand-name Lunesta, is a nonbenzodiazepine hypnotic drug used to treat insomnia. It is the active stereoisomer of zopiclone, belonging to the class of drugs known as cyclopyrrolones. Cyclopyrrolone drugs demonstrate high efficacy and low toxicity, offering a safer alternative to other drugs used for insomnia.
One major benefit of eszopiclone is that it is approved by the FDA for the long-term treatment of insomnia. This sets it apart from many other hypnotic sedatives, which are generally approved only for the relief of short-term (6-8 weeks) insomnia. Eszopiclone was initially approved by the FDA in 2004.
Definition
ChEBI: The (5S)- (active) enantiomer of zopiclone. Unlike almost all other hypnotic sedatives, which are approved only for the relief of short-term (6-8 weeks) insomnia, eszopiclone is approved by the U.S. Food and Drug Administration for long-te m use.
brand name
(Pharmacia & Upjohn); Ortho-EST (Sun)Lunesta (Sepracor).
Mechanism of action
The cyclopyrrole zopiclone is described as a “superagonist” at BZRs with the subunit composition α1β2γ2 and α1β2γ3, because it potentiates the GABA-gated current more than the benzodiazepine (flunitrazepam) reference agonist. Racemic zopiclone has been available in Europe since 199,2 and the higher affinity S-enantiomer (eszopiclone) was marketed in the United States in 2005, primarily to treat insomnia, because of its rapid onset and moderate duration (half-life, ~6 hours) of hypnotic-sedative effect. Less than 10% of orally administered eszopiclone is excreted unchanged, because it undergoes extensive CYP3A4- and CYP2E1-catalyzed oxidation and demethylation to metabolites excreted primarily in urine. "
Pharmacokinetics
Eszopiclone rapidly induces sleep and decreases sleep latency. It also aids in the maintenance of sleep, preventing frequent awakenings. This drug has shown anticonvulsant and muscle relaxant properties in animals but is used in humans for its sedating effects.
Eszopiclone is a central nervous system depressant with various effects. These include changes in alertness and motor coordination and the risk of next morning impairment, increasing with the amount of eszopiclone administered. Exercise caution and advise against driving a motor vehicle or activities that require full mental alertness the next morning. Complex sleep behaviors may result from eszopiclone use. Eszopiclone should be discontinued in these cases. Avoid the use of alcohol and other CNS depressants when eszopiclone is administered. Advise patients to skip the eszopiclone dose if alcohol has been consumed before bed or during the evening. Use the smallest dose of eszopiclone as possible, especially in elderly patients, who may experience exaggerated drug effects. Though the potential for dependence and abuse with eszopiclone is lower than for other hypnotic drugs, this drug has been abused and is known to cause dependence.
Synthesis
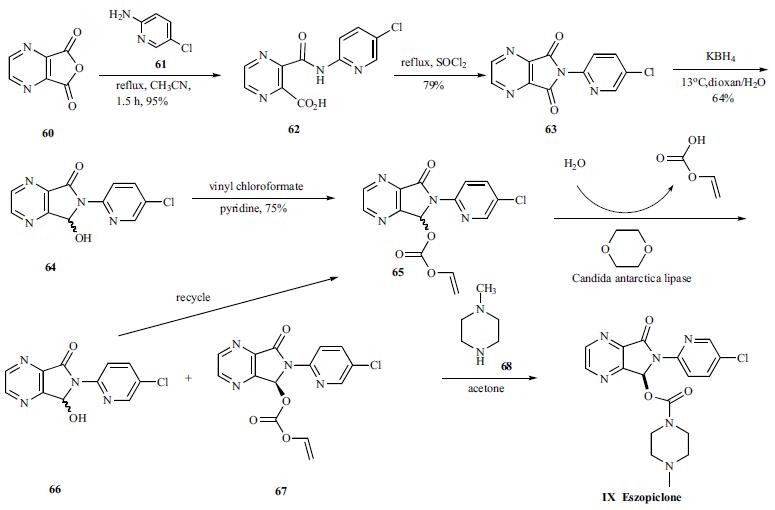
The synthesis of eszopicolone involves enzymatic resolution of a zopicolone derivative to give the chiral compound as depicted in the Scheme 12. Pyrazine-2,3- dicarboxylic acid anhydride was reacted with 2-amino- 5-chloropyridine (61) in refluxing acetonitrile to generate 3- (5-chloro-2-pyridyl)carbamoyl pyrazine-2-carboxylic acid (62) in 95% yield. Compound 62 was cyclized by treating with refluxing SOCl2 to give 6-(5-chloropyrid-2-yl)-5,7- dioxo-5,6-dihydropyrrolo[3,4-b]pyrazine (63) in 79% yield.Compound 63 was subjected to partial reduction with KBH4 in dioxane-water at low temperature to give 6-(5-chloro-2- pyridyl)-7-hydroxy-5,6-dihydropyrrolo[3,4-b]pyrazin-5-one (64) in 64% yield, which was esterified with vinyl chloroformate in pyridine to give corresponding vinyl acetate 65 in 75% yield. The racemic 65 was then subjected to kinetic resolution by a highly enantioselective enzymatic hydrolysis process. Chiral vinyl acetate 67 with desired stereochemistry was obtained when candida antarctica lipase was employed for hydrolysis of 65 in dioxane/water at 60oC for 2 days. Interestingly, the enzymatic hydrolysis stopped at 50% conversion and the hydrolyzed alcohol was recovered as the starting substrate 65 because of spontaneous racemization of the alcohol in the reaction medium. Therefore, although a maximum yield of kinetic resolution is 50%, the overall efficiency of this enzymatic process is 100% because of substrate recycling. Finally, the chiral vinyl acetate 67 was condensed with methyl piperazine in acetone to give eszopicolone (IX).
Metabolism
Following oral administration, eszopiclone is extensively biotransformed and the major metabolites are S-desmethylzopiclone and zopiclone-N-oxide, which are largely inactive.. The enzymes involved in the metabolism of eszopiclone are CYP3A (the primary metabolizing enzyme), CYP2C8, and CYP2E1. The N-oxide derivative shows weak pharmacological activity in animals. The N-desmethyl metabolite is pharmacologically active.
Advantage
The primary advantage of eszoplicone is that it has been shown to be effective in chronic insomnia (long-term treatment) in measures of sleep latency, total sleep time, and wake time after sleep onset without development of tolerance. Eszoplicone would appear to be most effectively used for patients who tend to awaken during the night rather than patients for whom the primary problem is initiating sleep.
Properties of Eszopiclone
| Melting point: | 202-204°C |
| Boiling point: | 580.7±50.0 °C(Predicted) |
| alpha | D20 +135 ±3° (c = 1.0 in acetone) |
| Density | 1.54±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly, Heated), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 6.70±0.10(Predicted) |
| color | White to Light Beige |
| InChI | InChI=1S/C17H17ClN6O3/c1-22-6-8-23(9-7-22)17(26)27-16-14-13(19-4-5-20-14)15(25)24(16)12-3-2-11(18)10-21-12/h2-5,10,16H,6-9H2,1H3/t16-/m0/s1 |
| CAS DataBase Reference | 138729-47-2(CAS DataBase Reference) |
Safety information for Eszopiclone
Computed Descriptors for Eszopiclone
| InChIKey | GBBSUAFBMRNDJC-INIZCTEOSA-N |
| SMILES | N1(C(O[C@H]2C3=NC=CN=C3C(=O)N2C2=NC=C(Cl)C=C2)=O)CCN(C)CC1 |
Eszopiclone manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 138729-47-2 Eszopiclone 98%View Details
138729-47-2 Eszopiclone 98%View Details
138729-47-2 -
 138729-47-2 98%View Details
138729-47-2 98%View Details
138729-47-2 -
 Eszopiclone 98%View Details
Eszopiclone 98%View Details
138729-47-2 -
 138729-47-2 Eszopiclone 98%View Details
138729-47-2 Eszopiclone 98%View Details
138729-47-2 -
 138729-47-2 Eszopiclone 99%View Details
138729-47-2 Eszopiclone 99%View Details
138729-47-2 -
 Eszopiclone 98%View Details
Eszopiclone 98%View Details
138729-47-2 -
 Eszopiclone 98%View Details
Eszopiclone 98%View Details
138729-47-2 -
 138729-47-2 98%View Details
138729-47-2 98%View Details
138729-47-2
