Zopiclone
Synonym(s):4-Methyl-1-piperazinecarboxylic acid 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester;Imovane
- CAS NO.:43200-80-2
- Empirical Formula: C17H17ClN6O3
- Molecular Weight: 388.81
- MDL number: MFCD00133931
- EINECS: 256-138-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
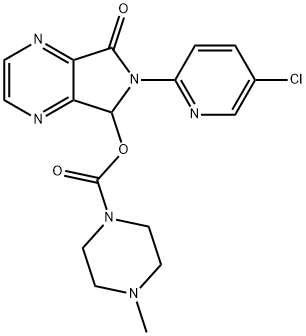
What is Zopiclone?
Absorption
Rapidly absorbed following oral administration.
Toxicity
Rare individual instances of fatal outcomes following overdose with racemic zopiclone have been reported in European postmarketing reports, most often associated with overdose with other CNS-depressant agent. Signs and symptoms of overdose effects of CNS depressants can be expected to present as exaggerations of the pharmacological effects noted in preclinical testing.
Description
Zopiclone is an effective hypnotic agent with a short duration of action. Although it interacts with the benzodiazepine receptor complex, it is reported to have minimal effects on memory, little synergy with alcohol, and low abuse potential.
Chemical properties
Crystalline Solid
Originator
Rhone-Poulenc (France)
The Uses of Zopiclone
Cyclopyrrolone member of a family of non-benzodiazepine GABAA receptor agonists. This is a controlled substance (depressant) in the US but not in Canada. Sedative, hypnotic
The Uses of Zopiclone
antihyperammonemic,antineoplastic
The Uses of Zopiclone
inhibits tyrosinase and prevents melanin formation used to whiten and lighten skin
Background
Zopiclone is a novel hypnotic agent used in the treatment of insomnia. Its mechanism of action is based on modulating benzodiazepine receptors. In addition to zopiclone's benzodiazepine pharmacological properties it also has some barbiturate-like properties.
Indications
For the short-term treatment of insomnia.
Definition
ChEBI: A pyrrolo[3,4-b]pyrazine compound having a 4-methylpiperazine-1-carboxyl group at the 5-position, a 5-chloropyridin-2-yl group at the 6-position and an oxo-substituent at the 7-position.
Manufacturing Process
Producing of 6-(5-chloropyrid-2-yl)-5-(4-methylpiperazin-1-yl)-carbonyloxy-7-
oxo-5,6-dihydropyrrolo[3,4-b]pyrazine by two methods.
1). A solution of 6-(5-chloropyrid-2-yl)-5-hydroxy-7-oxo-5,6-
dihydropyrrolo[3,4-b]pyrazine (12.0 g) in anhydrous dimethylformamide (360
ml) is added to a suspension of sodium hydride (50% dispersion in mineral
oil) (2.4 g) in anhydrous dimethylformamide (60 ml), whilst maintaining the
temperature at about -10°C. When the evolution of gas has ceased, a solution
of 1-chlorocarbonyl-4-methylpiperazine (8.1 g) in anhydrous
dimethylformamide (20 ml) is added, whilst maintaining the temperature at
about -10°C. The reaction mixture is stirred for a further 3 h whilst allowing it
to heat up gradually to a temperature of about 20°C, and then it is poured
into ice-water (1540 ml). The product which crystallizes is filtered off, washed
with water (150 ml) and then with diisopropyl ether (100 ml). After drying, a
product is obtained and is dissolved in ethyl acetate (600 ml). The solution
obtained is filtered through silica gel (250.0 g). Elution is then carried out with
ethyl acetate (3200 ml) followed by a mixture of ethyl acetate and methanol
The eluates are combined and concentrated to dryness under reduced
pressure. So 8.3 g of the 6-(5-chloropyrid-2-yl)-5-(4-methylpiperazin-1-yl)-
carbonyloxy-7-oxo-5,6-dihydropyrrolo[3,4-b]pyrazine are obtained, melting
point 178°C (recrystallisation from a mixture of acetonitrile and diisopropyl
ether 1:1; 190 ml).
2). 1-Methylpiperazine (155.0 g) is added to a suspension of 6-(5-chloropyrid-
2-yl)-7-oxo-5-phenoxycarbonyloxy-5,6-dihydropyrrolo[3,4-b]pyrazine (194.0
g) in acetone (970 ml) cooled to a temperature of about 3°C. The reaction
mixture is stirred for 3 h at a temperature of about 3°C and is then poured
into water (5000 ml). The product which precipitates is filtered off and then
washed with water (600 ml) and dried. This product is treated with methylene
chloride (1100 ml) at a temperature of about 20°C. The insoluble material is
filtered off and then the filtrate is washed with 1 N sodium hydroxide solution
(3x200 ml) and with water (3x200 ml). The organic phase is treated with
decolorizing charcoal (10.0 g), dried over potassium carbonate, filtered and
then concentrated to dryness under reduced pressure. The oily residue
obtained is dissolved in boiling acetonitrile (500 ml). The 101.0 g of 6-(5-
chloropyrid-2-yl)-5-(4-methylpiperazin-l-yl)carbonyloxy-7-oxo-5,6-
dihydropyrrolo[3,4-b]-pyrazine are obtained, melting point 178°C (washed
with ice cold acetonitrile, 50 ml, and then crystallizes with diisopropyl ether,
50 ml).
brand name
IMOVANE
Therapeutic Function
Sedative, Hypnotic
World Health Organization (WHO)
Zopiclone was introduced as a sedative in 1985. It remains registered in several countries and the World Health Organization is not aware of any other country that has refused registration.
Pharmacokinetics
Zopiclone is a nonbenzodiazepine hypnotic from the pyrazolopyrimidine class and is indicated for the short-term treatment of insomnia. While Zopiclone is a hypnotic agent with a chemical structure unrelated to benzodiazepines, barbiturates, or other drugs with known hypnotic properties, it interacts with the gamma-aminobutyric acid-benzodiazepine (GABABZ) receptor complex. Subunit modulation of the GABABZ receptor chloride channel macromolecular complex is hypothesized to be responsible for some of the pharmacological properties of benzodiazepines, which include sedative, anxiolytic, muscle relaxant, and anticonvulsive effects in animal models. Zopiclone binds selectively to the brain alpha subunit of the GABA A omega-1 receptor.
Clinical Use
Hypnotic
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism inhibited by
erythromycin; concentration significantly reduced by
rifampicin.
Antipsychotics: enhanced sedative effects.
Antivirals: concentration possibly increased by
ritonavir.
Metabolism
Extensively metabolized in the liver via decarboxylation (major pathway), demethylation, and side chain oxidation. Metabolites include an N-oxide derivative (weakly active; approximately 12% of a dose) and an N-desmethyl metabolite (inactive; approximately 16%). Approximately 50% of a dose is converted to other inactive metabolites via decarboxylation. Hepatic microsomal enzymes are apparently not involved in zopiclone clearance.
Metabolism
Zopiclone is extensively metabolised in the liver via the cytochrome P450 isoenzyme CYP3A4 and, to a lesser extent, CYP2C8; the 2 major metabolites, the less active zopiclone N-oxide and the inactive N-desmethylzopiclone, are excreted mainly in the urine. About 50
% of a dose is converted by decarboxylation to inactive metabolites, which are partly eliminated via the lungs as carbon dioxide.
Properties of Zopiclone
| Melting point: | 1780C |
| Boiling point: | 580.7±50.0 °C(Predicted) |
| Density | 1.1105 (estimate) |
| Flash point: | 2℃ |
| storage temp. | Store at RT |
| solubility | DMSO: 2 mg/mL |
| pka | pKa ﹣1.5±0.1(10% ACN in
aq. H2SO4
t = 25.0) (Uncertain) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 43200-80-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Zopiclone(43200-80-2) |
Safety information for Zopiclone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Zopiclone
| InChIKey | GBBSUAFBMRNDJC-UHFFFAOYSA-N |
Zopiclone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 Zopiclone solution CAS 43200-80-2View Details
Zopiclone solution CAS 43200-80-2View Details
43200-80-2 -
 Zopiclone 7 .5mg, For Insomnia, TabletView Details
Zopiclone 7 .5mg, For Insomnia, TabletView Details
43200-80-2 -
 Zopiclone 7 5mg, Non prescription, Treatment: InsomaniaView Details
Zopiclone 7 5mg, Non prescription, Treatment: InsomaniaView Details
43200-80-2 -
 Zopiclone 7 5mgView Details
Zopiclone 7 5mgView Details
43200-80-2 -
 Zopiclone 7 5mg tablets, For ClinicalView Details
Zopiclone 7 5mg tablets, For ClinicalView Details
43200-80-2 -
 7 .5 Mg ZOP Zopiclone TabletView Details
7 .5 Mg ZOP Zopiclone TabletView Details
43200-80-2 -
 7 5mg Zopicon Zopiclone TabletsView Details
7 5mg Zopicon Zopiclone TabletsView Details
43200-80-2 -
 Intas Zopiclone 7 5mg, TabletView Details
Intas Zopiclone 7 5mg, TabletView Details
43200-80-2
