Vortioxetine hydrobromide
- CAS NO.:960203-27-4
- Empirical Formula: C18H23BrN2S
- Molecular Weight: 379.36
- MDL number: MFCD22383961
- EINECS: 120-321-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Vortioxetine hydrobromide?
The Uses of Vortioxetine hydrobromide
Vortioxetine Hydrobromide is a multimodal serotonergic agent that inhibits 5-HT1A, 5-HT1B, 5-HT3A, 5-HT7 receptor and SERT (1,2,3).
What are the applications of Application
Vortioxetine Hydrobromide is a multimodal serotonergic agent and SR-1A (5-HT1A), SR-1B (5-HT1B), SR-3A (5-HT3A), SR-7 (5-HT7 receptor) and ST (SERT) inhibitor
Definition
ChEBI: Vortioxetine hydrobromide is a hydrobromide obtained by combining vortioxetine with one molar equivalent of hydrobromic acid. Used for treatment of major depressive disorder. It has a role as an antidepressant, an anxiolytic drug, a serotonergic antagonist and a serotonergic agonist. It contains a vortioxetine(1+).
Biological Activity
vortioxetine (lu aa21004) hbr (vortioxetine) is an antidepressant, [1] [2] and a serotonin (5-ht) receptor modulator [1] [3] with ki values of 3.7 nm, 19 nm, 200 nm, 33 nm, 15 nm, and 1.6 nm, to human (h) 5-ht3a receptor, h5-ht7 receptor, rat (r) 5-ht7 receptor, h5-ht1b receptor, h5-ht1a receptor and h5-ht transporter (hsert), respectively, with an ic50 value of 2080 nm to r5-ht7 receptor, and with an ec50 value of 460 nm to h5-ht1b receptor [3].5-ht is important in brain, acting as a neurotransmitter in neuromodulation [4].in hela cells expressing h5-ht1b receptors stably, lu aa21004 displayed a partial agonistic response with an ec50 value of 460±110 nm and an ia of 22%. in a polyclonal hek-293 cell line expressing the r5-ht7 receptor, lu aa21004 bound to the r5-ht7 receptor with a ki value of 200±27 nm and was a functional antagonist at the r5-ht7 receptor with an ic50 value of 2080±18 nm [3].in rats, subcutaneous (sc) administration of lu aa21004 resulted in ed50 values of 0.4 mg/kg and 3.2 mg/kg to the rsert and the r5-ht1b receptor, respectively. lu aa21004 acted as an antagonist of 5-ht3 receptor with an ed50 value of 0.11 mg/kg sc [3]. treatment with vortioxetine at doses of 2.5 and 5 mg/kg prior to the acquisition of novel objects made rats show 29 and 33 s, respectively, as average exploration values [5].
Synthesis
As the picture 2 showed, we have developed a new and practical synthetic route to vortioxetine hydrobromide on a hectogram scale. Adopting commercially available materials, through simple and traditional steps, including nucleophilic substitution, catalytic hydrogenation, and cyclization of the piperazine ring, gave the final product in 63.2% yield over three steps and with 99.3% purity (HPLC). Purification methods for the intermediates involved in the route are also given.
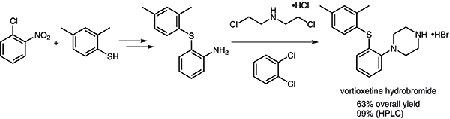
Pic.3 A new synthetic route to vortioxetine hydrobromide
Solubility in water
Vortioxetine hydrobromide is slightly soluble in water; at ambient temperature solubility is equivalent to approximately 1.3 mg base/mL, pH being 5.5 in the saturated solution.
Mode of action
Vortioxetine Hydrobromide is a hydrobromide salt form of vortioxetine, a serotonin (5-HT) modulator and stimulator (SMS), with antidepressant activity. Vortioxetine inhibits the reuptake of serotonin and norepinephrine from the synaptic cleft and acts variably as a serotonin receptor agonist (5-HT1A), partial agonist (5-HT1B) or antagonist (5-HT3, 5-HT1D and 5-HT7). It is not clear how this agent's purported multimodal mechanism of action contributes to its antidepressant effect; however, it is presumed to increase the synaptic availability of serotonin and norepinephrine.
Precautions
Vortioxetine is a serotonergic drug that has a risk of causing serotonin syndrome, especially when combined with other serotonergic drugs such as SSRIs, SNRIs, TCAs, or MAOIs.
Vortioxetine has been known to cause an increase in the risk of bleeding because of interference with serotonin reuptake. The concomitant use of NSAIDs, aspirin, warfarin, and anticoagulants may increase the risk of abnormal bleeding.
Among patients using vortioxetine, reports of symptoms of mania/hypo-mania were less than 0.1%. However, this drug should be used with caution in patients with a personal or family history of bipolar disorder, mania, or hypomania.
Hyponatremia was reported in one patient taking vortioxetine. Hyponatremia is related to the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Elderly patients are at higher risk for hyponatremia, as well as patients taking diuretics. Vortioxetine should be discontinued if signs and symptoms of hyponatremia are present and if there is a decrease in sodium levels[6].
References
[1]. connie sanchez, karen e. asin, francesc artigas, et al. vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. pharmacology & therapeutics, 2015, 145:43-57.
[2]. er-min gu, chengke huang, bingqing liang, et al. an uplc–ms/ms method for the quantitation of vortioxetine in rat plasma: application to a pharmacokinetic study. j. chromatogr. b, 2015, 997:70-74.
[3]. a. mørk, a. pehrson, l.t. brennum, s. møller nielsen, et al. pharmacological effects of lu aa21004: a novel multimodal compound for the treatment of major depressive disorder. j. pharmacol. exp. ther., 2012, 340:666-675.
[4]. david m. lovinger. serotonin’s role in alcohol’s effects on the brain. alcohol health & research world, 1997, 21(2):114-120.
[5]. arne mørk, liliana p. montezinho, silke miller, et al. vortioxetine (lu aa21004), a novel multimodal antidepressant, enhances memory in rats. pharmacology, biochemistry and behavior, 2013, 105:41-50.
[6] Andrew, et al. "Vortioxetine (brintellix): a new serotonergic antidepressant. "P & T : a peer-reviewed journal for formulary management(2015).
Properties of Vortioxetine hydrobromide
| Melting point: | >223°C (dec.) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Pale Beige to Light Beige |
| InChI | InChI=1S/C18H22N2S.BrH/c1-14-7-8-17(15(2)13-14)21-18-6-4-3-5-16(18)20-11-9-19-10-12-20;/h3-8,13,19H,9-12H2,1-2H3;1H |
Safety information for Vortioxetine hydrobromide
Computed Descriptors for Vortioxetine hydrobromide
| InChIKey | VNGRUFUIHGGOOM-UHFFFAOYSA-N |
| SMILES | S(C1C=CC(C)=CC=1C)C1C=CC=CC=1N1CCNCC1.Br |
Vortioxetine hydrobromide manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran



You may like
-
 Vortioxetine hydrobromide 98%View Details
Vortioxetine hydrobromide 98%View Details -
 Vortioxetine hydrobromide 98%View Details
Vortioxetine hydrobromide 98%View Details
960203-27-4 -
 Vortioxetine hydrobromide 960203-27-4 98%View Details
Vortioxetine hydrobromide 960203-27-4 98%View Details
960203-27-4 -
 960203-27-4 Vortioxetine hydrobromide 99%View Details
960203-27-4 Vortioxetine hydrobromide 99%View Details
960203-27-4 -
 960203-27-4 98%View Details
960203-27-4 98%View Details
960203-27-4 -
 Vortioxetine hydrobromide 960203-27-4 98%View Details
Vortioxetine hydrobromide 960203-27-4 98%View Details
960203-27-4 -
 Vortioxetine hydrobromide 98% CAS 960203-27-4View Details
Vortioxetine hydrobromide 98% CAS 960203-27-4View Details
960203-27-4 -
 Vortioxetine hydrobromide CAS 960203-27-4View Details
Vortioxetine hydrobromide CAS 960203-27-4View Details
960203-27-4
