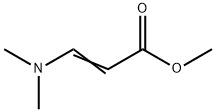
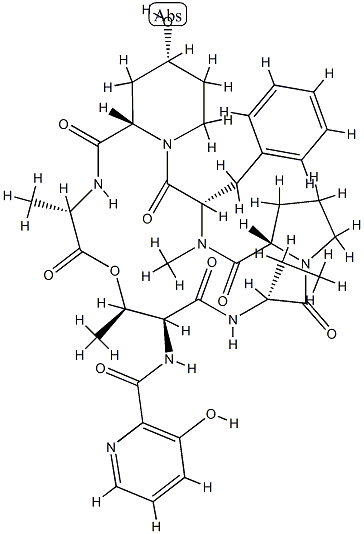
VIRGINIAMYCIN M1
Synonym(s):Mikamycin A;Staphylomycin
- CAS NO.:21411-53-0
- Empirical Formula: C28H35N3O7
- Molecular Weight: 525.59
- MDL number: MFCD00869411
- EINECS: 244-376-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
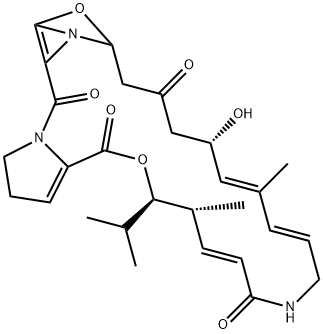
What is VIRGINIAMYCIN M1?
Chemical properties
Light yellow powder
The Uses of VIRGINIAMYCIN M1
Ostreogrycin A (virginiamycin M1, streptogramin A) is the major component of the virginiamycin complex. In the 1950s this complex was independently discovered so many times that the literature became highly confusing. Ostreogrycin A is a macrocyclic lactone antibiotic that acts synergistically with the structurally unrelated cyclic depsipeptides, virginiamycin B (ostreogrycin B, streptogramin B) and virginiamycin S, to inhibit peptide elongation. This is achieved by blocking formation of a peptide bond between the growing peptide chain (peptidyl-tRNA) linked to the 50S ribosome and aminoacyl-tRNA. Ostreogrycin A is highly active against Gram positive bacteria, particularly MRSA.
The Uses of VIRGINIAMYCIN M1
Ostreogrycin A (virginiamycin M1, streptogramin A) is the major component of the virginamycin complex. In the 1950s this complex was independently discovered so many times that the literature became highly confusing. Ostreogrycin A is a macrocyclic lactone antibiotic that acts synergistically with the structurally unrelated cyclic depsipeptides, virginiamycin B (ostreogrycin B, streptogramin B) and virginiamycin S, to inhibit peptide elongation. This is achieved by blocking formation of a peptide bond between the growing peptide chain (peptidyl-tRNA) linked to the 50S ribosome and aminoacyl-tRNA. Ostreogrycin A is highly active against Gram positive bacteria, particularly MRSA.
The Uses of VIRGINIAMYCIN M1
Macrolactone antibiotic. Antibacterial; growth promotant.
What are the applications of Application
Ostreogrycin A is a bacteriostatic inhibitor of translation at the 50S ribosome
Definition
ChEBI: A macrolide that is (together with pristinamycin IA) a component of pristinamycin, an oral streptogramin antibiotic produced by Streptomyces pristinaespiralis. Pristinamycin exhibits bactericidal activity against Gram positive organisms includ ng methicillin-resistant Staphylococcus aureus.
Biological Activity
virginiamycin m1 is a macrolide antibiotic that reversibly inhibits protein synthesis [1][2][3].virginiamycin complex contains two antibiotics, virginiamycin m1 and virginiamycin s1. streptogramins are divided into class a and class b based on their structures. virginiamycin m1, also known as streptogramin a, is a member of the streptogramin a group of antibiotics, which bind the 50s ribosomal subunit at the peptidyl transferase center to inhibit initiation and translocation. they show good bactericidal activity against methicillin-resistant s. aureus (mrsa), although resistance in mrsa is conferred by the cfr gene. virginiamycin m1 has activity against gram-positive and in select cases gram-negative bacteria. combination of group a and b streptogramins exhibit bactericidal activity [1]. virginiamycin m1 acted synergistically with virginiamycin s1 to irreversibly inhibit protein synthesis in bacteria. in cell-free systems, virginiamycin m1 and virginiamycin s1 bound to the large ribosomal subunit, and the affinity of ribosomes for vs is increased by vm [2][3].
Contact allergens
Pristinamycin is a systemic antibiotic of the synergistins/ streptogramins class, composed of two subunits: pristinamycin IA and pristinamycin IIA. It induces several types of drug reactions such as maculo-papular exanthema, systemic dermatitis, or acute generalized exanthematous pustulosis. Some patients have been previously skin-sensitized by virginiamycin. Crossreactivity is expected to virginiamycin and to the associated dalfopristin and quinupristin.
Contact allergens
Like the other streptogramin, pristinamycin, virginiamycin is made of two subunits, virginiamycin S1 and virginiamycin M1. Dermatitis was quite common in people using the formerly available topical virginiamycin. Occupational dermatitis was observed in the pharmaceutical industry, in breeders, and in a surgeon who used topical virginiamycin on postoperative wounds (personal observation).
References
[1]. fair rj, tor y. antibiotics and bacterial resistance in the 21st century. perspect medicin chem. 2014 aug 28;6:25-64.
[2]. kehrenberg c, cuny c, strommenger b, et al. methicillin-resistant and -susceptible staphylococcus aureus strains of clonal lineages st398 and st9 from swine carry the multidrug resistance gene cfr. antimicrob agents chemother. 2009 feb;53(2):779-81.
[3]. parfait r, cocito c. lasting damage to bacterial ribosomes by reversibly bound virginiamycin m. proc natl acad sci u s a. 1980 sep;77(9):5492-6.
Properties of VIRGINIAMYCIN M1
| Melting point: | 165-167℃ |
| Boiling point: | 825.2±65.0 °C(Predicted) |
| alpha | D20 -218° ( c = 0.34 in ethanol) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | chloroform/methanol: soluble10mg/mL |
| form | powder |
| pka | 13.18±0.70(Predicted) |
| color | yellow to tan |
| Stability: | Light Sensitive |
Safety information for VIRGINIAMYCIN M1
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for VIRGINIAMYCIN M1
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Virginiamycin M1 CAS 21411-53-0View Details
Virginiamycin M1 CAS 21411-53-0View Details
21411-53-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1