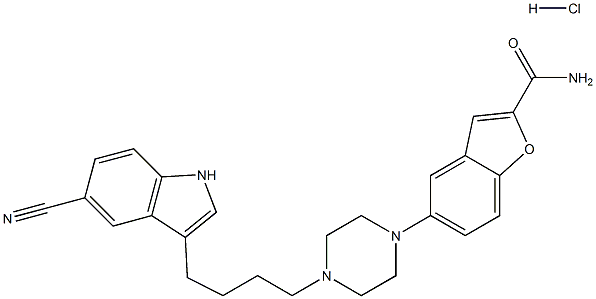
Vilazodone Hydrochloride
Synonym(s):5-[4-[4-(5-Cyano-1H-indol-3-yl)butyl]-1-piperazinyl]- 2-benzofurancarboxamide hydrochloride;EMD 68843;SB 659746A.
- CAS NO.:163521-08-2
- Empirical Formula: C26H28ClN5O2
- Molecular Weight: 477.98582
- MDL number: MFCD00940014
- EINECS: 695-883-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is Vilazodone Hydrochloride?
Description
Vilazodone is a selective serotonin reuptake inhibitor (SSRI) and a partial agonist of the serotonin (5-HT) receptor subtype 5-HT1A (IC50s = 0.2 and 0.5 nM, respectively). It increases extracellular 5-HT in the rat ventral hippocampus and frontal cortex when administered intraperitoneally at doses of 1 and 3 mg/kg. Vilazodone (1 mg/kg, i.p.) decreases immobility in the forced swim test in both rats and mice. Formulations containing vilazodone have been used in the treatment of depression.
The Uses of Vilazodone Hydrochloride
Vilazodone Hydrochloride, is the salt form of Vilazodone (V265000), which is a combined serotonin specific reuptake inhibitor (SSRI) and 5-HT1A receptor partial agonist currently under clinical evaluation for the treatment of major depression. Antidepressant.
Definition
ChEBI: A hydrochloride obtained by reaction of vilazodone with one equivalent of hydrochloric acid. Used for the treatment of major depressive disorder.
Clinical Use
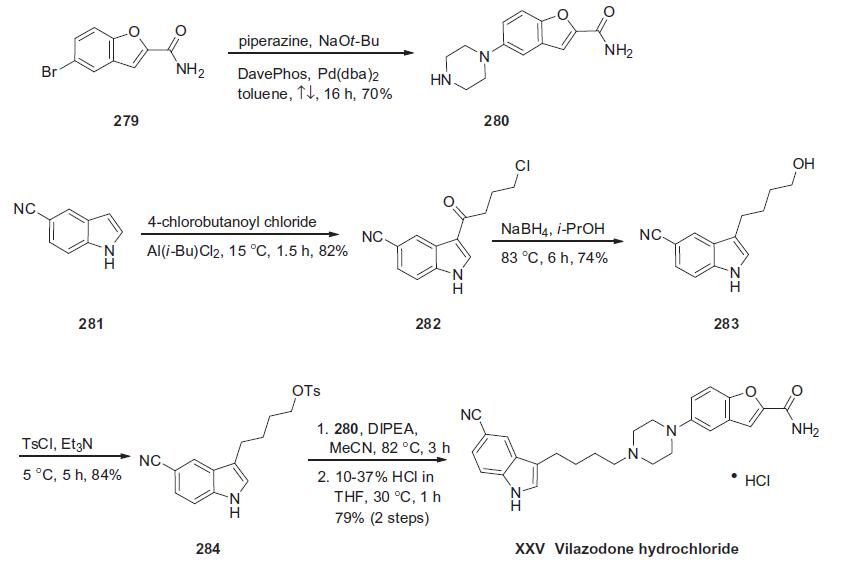
Althoughseveral synthetic approaches have beenreported,a process-scale synthesis of vilazodone consists of the union of an indole-containing butyl tosylate 284 with a benzofuranyl piperazine whose synthesis is described in the scheme below. Piperazine 280 arises from a Buchwald coupling of commercially available benzofuranyl bromide 279 with piperazine through the use of a unique catalyst system employing the DavePhos ligand. This single coupling step, which has been executed on multigram scale in 70% yield,210 circumvented the need for any protecting group chemistry for either the primary amide within 279 or the piperazine amine functionality. For the preparation of the key indole subunit, Friedel-Crafts acylation of commercially available 5-cyanoindole (281) proceeded in good yield at the 3-position of the indole with 4-chlorobutanoyl chloride in 82% yield. Treatment of the resulting chloroketone with sodium borohydride in refluxing isopropanol converted 282 to the corresponding terminal alcohol 283. Tosylation of this alcohol was followed by displacement with piperazine 280 to give vilazodone hydrochloride (XXV) after acidification.
Synthesis
Vilazodone hydrochloride is a combined serotonin reuptake inhibitor (SSRI) and 5-HT1A receptor partial agonist marketed under the trade name Viibryd.202 Viibryd was developed by Merck KGaA (Germany) and approved for the treatment of depression by the U.S. FDA on January 21st, 2011. Vilazodone has been shown to be well-tolerated at higher dosage levels, specifically by not causing significant weight gain or decreased sexual desire or function, which are improvements over existing antidepressant treatments.
storage
Store at -20°C
Properties of Vilazodone Hydrochloride
| Melting point: | 279°C(lit.) |
| Flash point: | 9℃ |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| Stability: | Hygroscopic |
Safety information for Vilazodone Hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Vilazodone Hydrochloride
Vilazodone Hydrochloride manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
Festiva Pharma
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran




You may like
-
 163521-08-2 Vilazodone Hydrochloride 98%View Details
163521-08-2 Vilazodone Hydrochloride 98%View Details
163521-08-2 -
 163521-08-2 99%View Details
163521-08-2 99%View Details
163521-08-2 -
 Vilazodone hydrochloride 95% CAS 163521-08-2View Details
Vilazodone hydrochloride 95% CAS 163521-08-2View Details
163521-08-2 -
 163521-08-2 Vilazodone hydrochloride 98%View Details
163521-08-2 Vilazodone hydrochloride 98%View Details
163521-08-2 -
 Vilazodone hydrochloride 163521-08-2 99%View Details
Vilazodone hydrochloride 163521-08-2 99%View Details
163521-08-2 -
 163521-08-2 98%View Details
163521-08-2 98%View Details
163521-08-2 -
 Vilazodone Hydrochloride CAS 163521-08-2View Details
Vilazodone Hydrochloride CAS 163521-08-2View Details
163521-08-2 -
 Vilazodone hydrochloride CAS 163521-08-2View Details
Vilazodone hydrochloride CAS 163521-08-2View Details
163521-08-2
