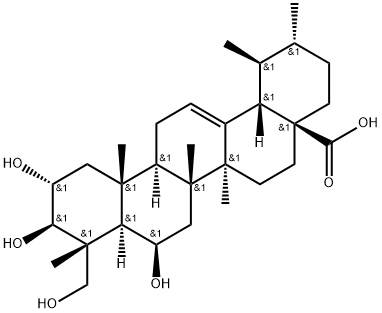
Ursonic acid
- CAS NO.:6246-46-4
- Empirical Formula: C30H46O3
- Molecular Weight: 454.68
- MDL number: MFCD09752413
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
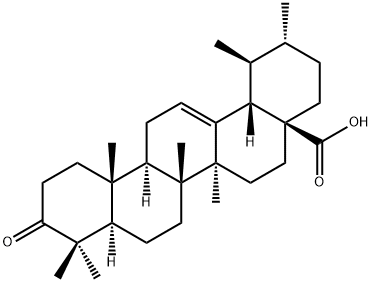
What is Ursonic acid?
Chemical properties
Ursonic acid (UNA; 3-Oxo-urs-12-en-28-oic acid) is a polycyclic triterpenoid. As the polarity of UNA is also low, ethanol and methanol, which are less polar than water, can dissolve UNA. Ursolic acid (ULA) and UNA commonly belong to ursane-type triterpenoids, and their basic structures are characterized by five rings, a double bond between C-12 and C-13, and a carboxyl group at the C-28 position. Both ULA and UNA hold three oxygen atoms, which can activate ligands and donate lone electron pairs to transition metals. The carboxyl group at the C-28 position significantly enhances the pharmacological potency of triterpenoids. The structure of UNA is quite similar to that of ULA, with UNA possessing a keto group at the C-3 position instead of the β-hydroxy group found in ULA. This difference in the C-3 position may explain the dissimilar biological activities of the two compounds[1].
Occurrence
Ursonic acid (UNA) can be found in several parts of various plants used in traditional herbal medicines. According to previous studies, UNA can be extracted from Crataegus pinnatifida and Malus baccata fruits. In particular, UNA is one of the primary compounds present in the ripened fruits of Ziziphus jujuba. It was reported that resins from Pistacia atlantica, Bursera delpechiana, and trees of the Dipterocarpaceae family contain triterpenoids, including UNA. UNA is also found in the roots of Toona sinensis, Piper betle, and Ficus microcarpa. Extraction of aerial parts of Lantana camara, which was traditionally used for the treatment of eczema, ulcers, rheumatism, and malaria, also resulted in the isolation of UNA. It is known that UNA can be obtained from the leaves of Lantana tiliaefolia and Rauvolfia vomitoria. Furthermore, UNA was isolated from a whole Catharanthus Roseus and Dracocephalum komarovi plant, which are widely used as folk medicines in Asia, Europe, and Africa. This extensive presence of UNA in medicinal herbs suggests that UNA itself can exert therapeutic potential against diseases such as cancer and infectious protozoa[1].
References
[1] Juhyeon Son, Sang Yeol Lee. “Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid.” Biomolecules (2020).
The Uses of Ursonic acid
Ursonic acid (UNA) is a phytochemical which can be also extracted from a great variety of traditional medicinal herbs. It can be used as a cosmetics additive and serve as a starting material for synthesis of more potent bioactive derivatives, such as experimental antitumor agents. Ursonic acid induces the apoptosis of human cancer cells through multiple signaling pathways.
Definition
ChEBI: Ursonic acid is a triterpenoid. It is a natural product found in Bursera linanoe, Lantana camara, and other organisms with data available.
Biological Activity
Ursonic acid is a natural triterpene acid that can induce apoptosis in human cancer cells through various signaling pathways.
Synthesis
3-oxo-12-en-28-ursolic acid (2) was obtained after compound 1 was refluxed in acetone under the action of Jones reagent for 5 hours, and then a series of anti-tumor Active novel quinoline oxadiazole derivatives.
Properties of Ursonic acid
| Melting point: | 284-285 °C |
| Boiling point: | 555.1±50.0 °C(Predicted) |
| Density | 1.09±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO : 10 mg/mL (21.99 mM; Need ultrasonic and warming) |
| pka | 4.68±0.70(Predicted) |
| form | powder to crystal |
| color | White to Almost white |
| CAS DataBase Reference | 6246-46-4 |
Safety information for Ursonic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Ursonic acid
| InChIKey | MUCRYNWJQNHDJH-OADIDDRXSA-N |
| SMILES | C1(=O)[C@@]([C@]2([C@](C)(CC1)[C@]1([H])[C@@]([C@]3(C(=CC1)[C@]1([C@@](CC[C@H]([C@@H]1C)C)(C(O)=O)CC3)[H])C)(C)CC2)[H])(C)C |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-Oxours-12-en-28-oic Acid CAS 6246-46-4View Details
3-Oxours-12-en-28-oic Acid CAS 6246-46-4View Details
6246-46-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
