oxprenolol
- CAS NO.:6452-71-7
- Empirical Formula: C15H23NO3
- Molecular Weight: 265.35
- MDL number: MFCD00243001
- EINECS: 2292579
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:41
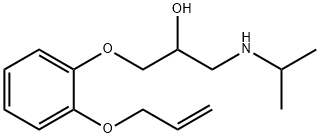
What is oxprenolol?
Absorption
Oral bioavailability is 20-70%.
Toxicity
Symptoms of overdose include abdominal irritation, central nervous system depression, coma, extremely slow heartbeat, heart failure, lethargy, low blood pressure, and wheezing.
Description
Oxprenolol induced contact dermatitis in a worker at a pharmaceutical plant, in the division for drug synthesis. Epichlorhydrin was also used for the production of both propranolol and oxprenolol.
Originator
Trasicor,Ciba Geigy ,Italy,1970
The Uses of oxprenolol
Oxprenolol is a free base of Oxprenolol Hydrochloride(O870500) which is a β-Adrenergic blocker. Antihypertensive, antianginal, antiarrhythmic.
The Uses of oxprenolol
Vasodilator(coronary).
Indications
Used in the treatment of hypertension, angina pectoris, arrhythmias, and anxiety.
Background
A beta-adrenergic antagonist used in the treatment of hypertension, angina pectoris, arrhythmias, and anxiety.
Definition
ChEBI: 1-(propan-2-ylamino)-3-(2-prop-2-enoxyphenoxy)-2-propanol is an aromatic ether.
Manufacturing Process
75 grams of pyrocatechol monoallyl ether, 75 grams of epichlorohydrin, 75 grams of potassium carbonate and 400 ml of acetone are stirred and heated at the boil for 12 hours. The potassium carbonate is then filtered off. The solvent is distilled off in a water-jet vacuum. The residual oil is dissolved in ether and agitated with 2 N sodium hydroxide solution. The ether is separated, dried and distilled off. The residue is distilled in a water-jet vacuum. 3-(ortho-allyloxy-phenoxy)-1,2-epoxypropane passes over at 145° to 157°C under 11 mm Hg pressure. A solution of 15 grams of 3-(ortho-allyloxyphenoxy)-1,2-epoxypropane and 15 grams of isopropylamine in 20 ml of ethanol is refluxed for 4 hours. The excess amine and the alcohol are then distilled off under vacuum, to leave 1-isopropylamino-2-hydroxy-3-(orthoallyloxy-phenoxy)-propane which melts at 75° to 80°C after recrystallization from hexane.
brand name
Trasicor (Novartis).
Therapeutic Function
Antiarrhythmic
Contact allergens
The beta-blocker oxprenolol induced contact dermatitis in a worker at a pharmaceutical plant, in a division for drug synthesis. Epichlorhydrin was also used for the production of drugs propranolol and oxprenolol.
Pharmacokinetics
Oxprenolol is a non-selective beta blocker with some intrinsic sympathomimetic activity. Oxprenolol is a lipophilic molecule and hence, it is able to cross the blood-brain barrier. As such, it is associated with a higher incidence of CNS-related side effects than hydrophilic ligands such as atenolol, sotalol and nadolol. Oxprenolol is an potent beta-blocker and should not be administered to asthmatics because it can cause irreversible airway failure and inflammation.
Metabolism
Hepatic.
Properties of oxprenolol
| Melting point: | 78-80° |
| Boiling point: | 408.57°C (rough estimate) |
| Density | 1.0479 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| solubility | Dichloromethane (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 13.89±0.20(Predicted) |
| color | White to Off-White |
| Stability: | Stable under recommended storage conditions., Stable Under Recommended Storage C |
Safety information for oxprenolol
Computed Descriptors for oxprenolol
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4