Arbutin
Synonym(s):p-Arbutin;4-Hydroxyphenyl-β-D -glucopyranoside;Hydroquinone β-D -glucopyranoside
- CAS NO.:497-76-7
- Empirical Formula: C12H16O7
- Molecular Weight: 272.25
- MDL number: MFCD00016915
- EINECS: 207-850-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
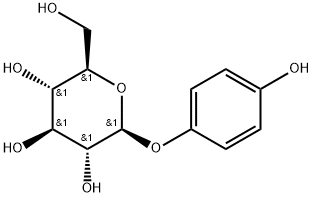
What is Arbutin?
Absorption
Arbutin was found to be extensively absorbed from the gastrointestinal tract where it is primarily converted to hydroquinone .
Toxicity
In an acute oral toxicity study, the LD50-value for β-arbutin is 9804 mg/kg bw for the mouse and 8715 mg/kg bw for the rat . Dermal LD50 value in rat and mouse was reported to be greater than 928 mg/kg bw, according to an acute dermal toxicity study . Extremely high doses may cause ringing in the ears, shortness of breath, convulsions, collapse, vomiting and delirium . Nausea and vomiting were seen individuals with sensitive stomachs following oral ingestion of 15 g of dried uva ursi leaves that contain arbutin .
Description
Arbutin is a β-D-glucopyranoside HQ derivative and a plant-derived compound found in the dried leaves of several plant species, including blueberry, cranberry, bearberry, and pear trees. It suppresses tyrosinase activity without altering RNA expression.
Description
Arbutin is a glycosylated hydroquinone that has been found in Arctostaphylos plants and has diverse biological activities, including tyrosinase inhibitory, antioxidant, and anti-inflammatory properties. It inhibits human tyrosinase activity in crude tyrosinase solution isolated from human melanocytes (IC50s = 5.7 and 18.9 mM using L-tyrosine and L-DOPA as substrates, respectively) as well as in intact melanocytes (IC50 = 0.5 mM). Arbutin (50 μM) inhibits hemolysis induced by the free radical generator AAPH in sheep erythrocytes and inhibits AAPH-induced decreases in cell viability in cultured human skin fibroblasts when used at concentrations greater than 125 μM. In an LPS-induced rat model of acute lung injury, arbutin (50 mg/kg) prevents increases in IL-1β, IL-6, and TNF-α levels in lung tissue and serum. Formulations containing arbutin have been used in the treatment of hyperpigmentation disorders.
Chemical properties
Arbutin is also known as hydroquinone glucoside and has two optical isomers, α and ?, with the latter having biological activity. At room temperature, it appears as a white powder with a slight yellowish tint that is soluble in water, methanol, ethanol, propylene glycol, and glycerin aqueous solutions without precipitation. However, it is insoluble in chloroform, ether, and petroleum ether.
Physical properties
Appearance: white powder. Solubility: soluble in hot water. Melting point: 198–201?°C
History
Arbutin is a hydroquinone compound with two epimers, α and β arbutin. The sources
of α-arbutin and β-arbutin are completely different. β-arbutin can be prepared by
plant extraction, plant cell culture, and artificial synthesis. Arbutin can relieve cough
and asthma and has whitening effect.
The Japanese cosmetics company Shiseido developed the arbutin as a whitening
agent in the 1990s. Arbutin can not only reduce skin freckles, senile plaques, and
chloasma but also relieve acne and improve healing after skin burns. Arbutin is the
epimer of β-arbutin, and the spatial orientation of their glycosidic bonds is just the
opposite. Alpha arbutin is generally prepared by different microbial enzymes. A
molecule of glucose and a molecule of hydroquinone combine to form a molecule
α-arbutin . Alpha arbutin improves ultraviolet burn scar. α-Arbutin can be used in
a variety of skin whitening cosmetics since it is chemically stable.
The Uses of Arbutin
Arbutin is a glycosylated hydroquinone extracted from bearberry plant. Arbutin is a known inhibitor of tyrosinase, which in turn prevents the formation of melanin. Arbutin is often used as a skin-ligh tening agent in cosmetic products.
The Uses of Arbutin
Antibacterial; tyrosinase inhibitor, depigmentor, antitussive
The Uses of Arbutin
veterinary drug
The Uses of Arbutin
arbutin is used primarily for its anti-oxidant and bleaching properties. Arbutin is the active constituent of bearberry, and found in other plant sources, including wheat. It acts as a tyrosinase inhibitor by converting to hydroquinone, and thus can prevent melanin formation.
Indications
Indicated for over-the-counter use for epidermal hyperpigmentation in various skin conditions, such as melasma, freckles, and senile lentigines.
What are the applications of Application
Arbutin is an inhibitor of Tyrosinase
Background
Extracted from the dried leaves of bearberry plant in the genus Arctostaphylos and other plants commonly in the Ericaceae family, arbutin is a beta-D-glucopyranoside of Hydroquinone. It is found in foods, over-the-counter drugs, and herbal dietary supplements . Most commonly, it is an active ingredient in skincare and cosmetic products as a skin-lightening agent for the prevention of melanin formation in various skin conditions that involve cutaneous hyperpigmentation or hyperactive melanocyte function . It has also been used as an anti-infective for the urinary system as well as a diuretic . Arbutin is available in both natural and synthetic forms; it can be synthesized from acetobromglucose and Hydroquinone . Arbutin is a competitive inhibitor of tyrosinase (E.C.1.14.18.1) in melanocytes , and the inhibition of melanin synthesis at non-toxic concentrations was observed in vitro. Arbutin was shown to be less cytotoxic to melanocytes in culture compared to Hydroquinone .
Definition
ChEBI: Arbutin also called Hydroquinone O-beta-D-glucopyranoside, is a monosaccharide derivative that is hydroquinone attached to a beta-D-glucopyranosyl residue at position 4 via a glycosidic linkage. It has a role as a plant metabolite and an Escherichia coli metabolite. It is a beta-D-glucoside and a monosaccharide derivative. It is functionally related to a hydroquinone.
Indications
Arbutin has bactericidal, anti-inflammatory, and whitening effects and is mainly used in whitening cosmetics.
Pharmacokinetics
At non-toxic concentrations, arbutin inhibited the activity of tyrosinase in cultured human keratinocytes, while having minimal effect on the expression of tyrosinase mRNA or the synthesis of the enzyme . α-Arbutin produced a concentration-dependent inhibition of melanin synthesis of human melanoma cells, HMV-II . No inhibitory effect on HMV-II cell growth was seen at concentrations lower than 1.0 mM. At concentrations of 0.5 mM of arbutin, tyrosinase activity was reduced to 60% of that in non-treated cells . The addition of arbutin blocked and inhibited α-MSH-stimulated melanogenesis in B16 melanoma cells, brownish guinea pig, and human skin tissue . In a pilot study of healthy male adults exposed to UV B irradiation, topical administration of arbutin inhibited UV-induced nuclear factor-kappaB activation in human keratinocytes . In mouse skin, arbutin counteracted oxidative stress induced by 12-O-tetradecanoylphorbol-13-acetate .
Pharmacology
Arbutin could effectively inhibit the activity of tyrosinase in skin cells and block the formation of melanin without affecting cell proliferation . Furthermore, it could accelerate the decomposition and excretion of melanin and thereby reduce skin pigmentation and eliminate freckles. In addition, arbutin shows no toxicity, irritation, sensitization, and other side effects . Alpha arbutin is safer and has a stronger inhibitory effect on tyrosinase. At present the whitening cosmetics market in the developed countries has been almost monopolized by arbutin.
Clinical Use
Arbutin is mainly used in high-level cosmetics and has been formulated into skin cream, freckle cream, and senior pearl cream. Arbutin is a major component of medicine for treating burn and scald, characterized by rapid elimination of pain and swelling and fast healing, leaving no scars. Arbutin can also be used as raw materials for intestinal anti-inflammatory drug, with sterilization, anti-inflammatory effect, and nontoxic side effects.
Metabolism
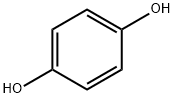
Arbutin is readily susceptible to hydrolysis in dilute acids to yield D-glucose and hydroquinone. It is expected that orally administered arbutin is easily hydrolyzed to free hydroquinone molecules by stomach acid . Hydroquinone is further metabolized into the main metabolites, hydroquinone glucuronide and hydroquinone sulfate .
Purification Methods
The glycoside from Protea exima is purified by recrystallisation from H2O or moist EtOAc (as monohydrate), after chromatography through silica Gel using EtOAc/MeOH. Crystallisation from EtOH/CHCl3 gives crystals m 199-200o with intermediate melting at 164o and resolidifying. The pentaacetate crystallises from EtOH in fine needles with m 145-146o, [] D 20 -28.2o (c 2, Me2CO). [Robinson & Waters J Chem Soc 2729 1930, IR, NMR, MS: Perold et al. J Chem Soc, Perkin Trans 1 239 1979, Beilstein 17/7 V 110.]
Properties of Arbutin
| Melting point: | 195-198 °C |
| Boiling point: | 375.31°C (rough estimate) |
| alpha | -64 º (c=3) |
| Density | 1.3582 (rough estimate) |
| refractive index | -65.5 ° (C=4, H2O) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 50 mg/mL hot, clear |
| form | neat |
| pka | 10.10±0.15(Predicted) |
| form | Solid |
| color | White |
| optical activity | [α]/D -64.0±2.0°, c = 3 in H2O |
| Water Solubility | 10-15 g/100 mL at 20 ºC |
| Sensitive | Hygroscopic |
| Merck | 14,773 |
| BRN | 89673 |
| Stability: | Stable. Hygroscopic - store under dry nitrogen. |
| CAS DataBase Reference | 497-76-7(CAS DataBase Reference) |
| EPA Substance Registry System | .beta.-D-Glucopyranoside, 4-hydroxyphenyl (497-76-7) |
Safety information for Arbutin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Arbutin
| InChIKey | BJRNKVDFDLYUGJ-RMPHRYRLSA-N |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran



You may like
-
 Arbutin CAS 497-76-7View Details
Arbutin CAS 497-76-7View Details
497-76-7 -
 Arbutin CAS 497-76-7View Details
Arbutin CAS 497-76-7View Details
497-76-7 -
 Arbutin CAS 497-76-7View Details
Arbutin CAS 497-76-7View Details
497-76-7 -
 Arbutin 98% CAS 497-76-7View Details
Arbutin 98% CAS 497-76-7View Details
497-76-7 -
 Arbutin CAS 497-76-7View Details
Arbutin CAS 497-76-7View Details
497-76-7 -
 Arbutin CAS 497-76-7View Details
Arbutin CAS 497-76-7View Details
497-76-7 -
 Arbutin CAS 497-76-7View Details
Arbutin CAS 497-76-7View Details
497-76-7 -
 609-15-4View Details
609-15-4View Details
609-15-4
