U0126-EtOH
- CAS NO.:1173097-76-1
- Empirical Formula: C20H22N6OS2
- Molecular Weight: 426.56
- MDL number: MFCD25977152
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
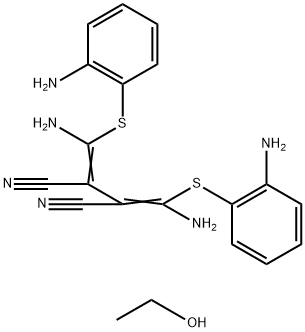
What is U0126-EtOH?
The Uses of U0126-EtOH
A chemically synthesized and highly selective inhibitor of both MEK1 and MEK2 with IC50s of 70 nM and 60 nM, respectively.
Definition
ChEBI: U0126.EtOH is an addition compound obtained by combining equimolar amounts of (2Z,3Z)-bis{amino[(2-aminophenyl)sulfanyl]methylidene}butanedinitrile (U0126) and ethanol. An inhibitor of mitogen-activated protein kinase that also exhibits anti-cancer properties. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, an apoptosis inducer, an antineoplastic agent, an antioxidant, an osteogenesis regulator and a vasoconstrictor agent. It contains an U0126.
Biological Activity
u0126-etoh is a selective inhibitor of mek1 and mek2 with ic50 values of 70 nm and 60 nm, resepctively [1].u0126 was screened out as an anti-inflammatory agent that inhibited ap-1 transcription with ic50 value of 1μm and had no interactions with gres. u0126 binds mek1/2 in a unique site. this inhibition of mek1/2 is noncompetitive with erk and atp. u0126 showed no effects on other mapkks. in ht22 cells, u0126 treatment significantly inhibited the cell injury caused by oxidative glutamate toxicity and remarkably blocked the phosphorylation of erk1/2. besides that, u0126 exerted no neuroprotection against other stimuli such as tnfα and actinomycin d. u0126 treatment also protected the primary cultured cortical neurons from oxidative glutamate toxicity and hypoxia/reoxygenation [1, 2].
References
[1] duncia j v, santella iii j b, higley c a, et al. mek inhibitors: the chemistry and biological activity of u0126, its analogs, and cyclization products. bioorganic & medicinal chemistry letters, 1998, 8(20): 2839-2844.
[2] satoh t, nakatsuka d, watanabe y, et al. neuroprotection by mapk/erk kinase inhibition with u0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. neuroscience letters, 2000, 288(2): 163-166.
Properties of U0126-EtOH
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | ≥21.33 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| form | solid |
Safety information for U0126-EtOH
Computed Descriptors for U0126-EtOH
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 U0126 ethanolate CAS 1173097-76-1View Details
U0126 ethanolate CAS 1173097-76-1View Details
1173097-76-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1