SB 202190
Synonym(s):4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole;4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole, FHPI, p38 MAP Kinase Inhibitor II;InSolution p38 MAP Kinase Inhibitor II;InSolution SB 202190 - CAS 152121-30-7 - Calbiochem;SB 202190 - CAS 152121-30-7 - Calbiochem
- CAS NO.:152121-30-7
- Empirical Formula: C20H14FN3O
- Molecular Weight: 331.34
- MDL number: MFCD00941964
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
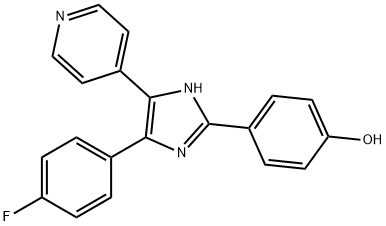
What is SB 202190?
Description
SB 202190 is a selective, potent, cell-
The Uses of SB 202190
SB 202190 is a DFG inhibitor used in the treatment of colorectal cancer. It is a highly selective, potent inhibitor of p38 MAP kinase. SB-202190 is able to promote proliferation of C6 cells in lower c oncentration but induced apoptosis in higher levels. Studies show that SB202190 can protect neural function from ischemia/reperfusion injury and regulate the activation of Bcl-2 and Bax protein after ischemia/reperfusion in rat.
The Uses of SB 202190
SB 202190 is a DFG inhibitor used in the treatment of colorectal cancer. It is a highly selective, potent inhibitor of p38 MAP kinase. SB-202190 is able to promote proliferation of C6 cells in lower concentration but induced apoptosis in higher levels. Studies show that SB202190 can protect neural function from ischemia/reperfusion injury and regulate the activation of Bcl-2 and Bax protein after ischemia/reperfusion in rat.
What are the applications of Application
SB 202190 is an inhibitor of the isoforms p38α and p38β
Definition
ChEBI: A member of the class of imidazoles that is 1H-imidazole in which the hydrogens at positions 2, 4, and 5 are replaced by 4-hydroxyphenyl, pyridin-4-yl, and 4-fluorophenyl groups, respectively. It is a widely used inhibitor of mitogen-act vated protein kinase (MAPK) alpha and beta.
General Description
A potent, reversible, competitive, and cell-permeable inhibitor of p38 MAP kinase. Inhibits p38 phosphorylation of myelin basic protein (MBP) with no effect on the activity of the ERK or JNK MAP kinase subgroups. Also inhibits the kinase activity of p38β (Ki = 16 nM; IC50 = 350 nM) and p38 phosphorylation of activating transcription factor 2 (ATF-2; IC50 = 280 nM). Blocks LPS-induced TNF-α and interleukin biosynthesis. Reported to induce LDL receptor expression in Hep52 cells. A 1 mg/ml solution of SB 202190 (Cat. No. 559397) in anhydrous DMSO is also available.
Biological Activity
A highly selective, potent and cell-permeable inhibitor of p38 MAP kinase. Binds within the ATP pocket of the active kinase (K d = 38 nM, as measured in recombinant human p38), and selectively inhibits the p38 α and β isoforms (IC 50 = 50 and 100 nM at SAPK2a/p38 and SAPK2b/p38 β 2 respectively). Also available as part of the MAPK Inhibitor Tocriset™ .
Biochem/physiol Actions
Cell permeable: yes
storage
-20°C
References
1) Young et al. (1997), Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site; J. Biol. Chem., 272 12116 2) Davies et al. (2000), Specificity and mechanism of action of some commonly used protein kinase inhibitors; Biochem. J., 351 95 3) Manthey et al. (1998), SB202190, a selective inhibitor of p38 mitogen-activated protein kinase, is a powerful regulator of LPS-induced mRBAs in monocytes; J. Leukoc. Biol., 64 409 4) Nemoto et al. (1998), Induction of apoptosis by SB202190 through inhibition of p38β mitogen-activated protein kinase; J. Biol. Chem., 273 16415
Properties of SB 202190
| Melting point: | 240-243℃ |
| Boiling point: | 565.7±50.0 °C(Predicted) |
| Density | 1.31 |
| RTECS | SL4584000 |
| storage temp. | 2-8°C |
| solubility | DMSO: 30 mg/mL, soluble |
| form | Pale yellow solid |
| pka | 9.14±0.15(Predicted) |
| color | pale yellow |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. |
Safety information for SB 202190
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for SB 202190
| InChIKey | QHKYPYXTTXKZST-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 SB 202190 98.00% CAS 152121-30-7View Details
SB 202190 98.00% CAS 152121-30-7View Details
152121-30-7 -
 SB 202190 CAS 152121-30-7View Details
SB 202190 CAS 152121-30-7View Details
152121-30-7 -
 SB 202190 CAS 152121-30-7View Details
SB 202190 CAS 152121-30-7View Details
152121-30-7 -
 SB 202190 CAS 152121-30-7View Details
SB 202190 CAS 152121-30-7View Details
152121-30-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1