Rapamycin
Synonym(s):Rapamycin;Sirolimus;23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine;23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine solution;mTOR Inhibitor I
- CAS NO.:53123-88-9
- Empirical Formula: C51H79NO13
- Molecular Weight: 914.17
- MDL number: MFCD00867594
- EINECS: 610-965-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
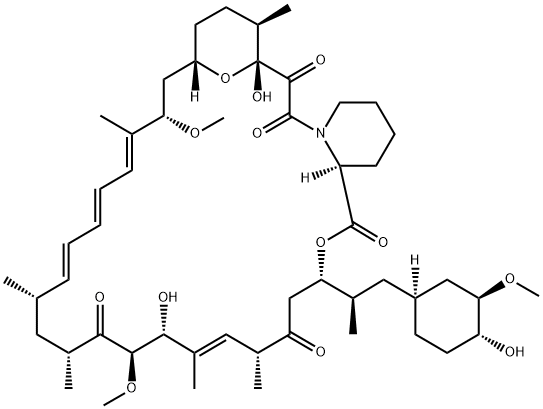
What is Rapamycin?
Absorption
In adult renal transplant patients with low- to moderate-immunologic risk, oral administration of 2 mg sirolimus led to a Cmax of 14.4 ± 5.3 ng/mL for oral solution and 15.0 ± 4.9 ng/mL for oral tablets. The tmax was 2.1 ± 0.8 hours for oral solution and 3.5 ± 2.4 hours for oral tablets. In healthy subjects, the tmax is one hour. In a multi-dose study, steady-state was reached six days following repeated twice-daily administration without an initial loading dose, with the average trough concentration of sirolimus increased approximately 2- to 3-fold. It is suspected that a loading dose of three times the maintenance dose will provide near steady-state concentrations within one day in most patients.
The systemic availability of sirolimus is approximately 14%. In healthy subjects, the mean bioavailability of sirolimus after administration of the tablet is approximately 27% higher relative to the solution. Sirolimus tablets are not bioequivalent to the solution; however, clinical equivalence has been demonstrated at the 2 mg dose level. Sirolimus concentrations, following the administration of Rapamune Oral Solution to stable renal transplant patients, are dose-proportional between 3 and 12 mg/m2.
Toxicity
Oral LD50 of sirolimus is 800 mg/kg in rats and 2500 mg/kg in mouse.
Sirolimus is a narrow therapeutic index drug. Although there are reports of overdose with sirolimus, there is limited information on overdose in the clinical setting. Symptoms of overdose are consistent with the adverse effects of sirolimus. General supportive measures are recommended in the event of an overdose. Because sirolimus has low aqueous solubility and high erythrocyte and plasma protein binding, it is not expected to be dialyzable to any significant extent.
Description
Macrolides, compounds that contain large (14–16-membered) lactone rings and reduced saccharide substituents, are natural products derived from bacteria. Many macrolides, such as erythromycin and azithromycin, have been formulated into commercial antibiotics.
One macrolide, rapamycin, has a long, convoluted history. In 1964, University of Montreal microbiologist Georges Nógrády, a member of a scientific expedition to Easter Island in the South Pacific, gathered soil samples on the island as part of his effort to discover why the natives did not contract tetanus in spite of favorable conditions for the bacterium.
After finding only one tetanus spore in more than five dozen samples, Nógrády gave the soil to natural product scientists at Ayerst Pharmaceuticals in Montreal. After years of painstaking work, these researchers found that a bacterium in the soil produced a fungicidal compound. Some years later, the molecule’s structure was elucidated, and it was named rapamycin. Eventually it also became known as sirolimus.
Wait—there’s more. Rapamycin turned out to have immunosuppressant and antiproliferative properties and was on its way to being developed as a commercial drug.
But in 1982, Ayerst underwent a restructuring, and the project was dropped. One thing led to another; and after the company became Wyeth-Ayerst, research was resumed.
More years went by, and, in 1999, rapamycin received US FDA approval as an immunosuppressant for organ transplants. A derivative was approved to treat kidney cancer in 2007. Then, in 2009, researchers at Jackson Laboratories in Maine discovered the drug’s “fountain of youth” attributes—it extended the lives of lab mice by 6 months or more.
Today, the main concern about using rapamycin (now owned by Pfizer) in humans is its now-undesirable immunosuppression activity. The research goes on.
Description
Rapamycin is a member of the macrolide immunosuppressant family and a FRAP inhibitor. Rapamycin exhibits binding and inhibitory actions to the FK506 binding protein (FKBP5) proline rotamase via simultaneous binding by FKBP12 and FRAP. FRAP (RAFT1) proteins exhibit homology to PI 4- and PI 3-kinases, which have PI 4-kinase and autophosphorylating activities. The rapamycin/FKBP complex does not inhibit the FRAP PI 4-kinase activity, but does inhibit FRAP autophosphorylation. Rapamycin is unique in its ability to inhibit lymphokine induced cell proliferation at the G1 and S phase as well as an irreversible cellular arrest at the G1 phase in S. cerevisiae cells. Rapamycin also exhibits selective signal blocking leading to the activation of p70/85 S6 kinase, which is potentially due to the inhibition of FRAP autophosphorylation or protein kinase activity. Rapamycin also exhibits Angiogenesis inhibition, possibly through the inhibition of the Akt pathway. Rapamycin is an inhibitor of mTOR. Rapamycin is also known as Rapamune, 23,27-Epoxy-3H-pyrido[2,1-c] oxaazacyclohentriacontine, AY 22989, and mTOR Inhibitor I.
Chemical properties
White to Off-White Solid
Originator
Rapamune,Wyeth Laboratories,UK
Occurrence
Rapamycin was first discovered in 1972 in the soil of Easter Island produced by a bacterium called Streptomyces hygroscopicus. It takes its name from Rapa Nui, the indigenous name for the island. It is known clinically as sirolimus or Rapamune.
Characteristics
Class: serine/threonine; Treatment: organ rejection, LAM; Other name: Sirolimus, AY-22989; Oral bioavailability = 14%; Elimination half-life = 63 h; Protein binding = 92%
The Uses of Rapamycin
Rapamycin is a triene macrolide discovered in 1995 as a metabolite of Streptomyces hygroscopicus found in a soil obtained on Rapi Nui (Easter Island). Rapamycin displayed potent and selective antifungal activity, notably against Candida albicans. Interest in the metabolite waned until the structural relationship to the potent immunosuppressant fujimycin (Antibiotic FK506) was recognised in the mid-1980s. This recognition led to the re-discovery of rapamycin as a highly selective antitumour and immunosuppressant. Rapamycin inhibits the activity of the protein, mTOR (mammalian target of rapamycin) which functions in a signalling pathway to promote tumour growth. Rapamycin binds to a receptor protein (FKBP12). The rapamycin/FKB12 complex then binds to mTOR and prevents interaction of mTOR with target proteins in this signalling pathway.
Rapamycin and its derivatives are inhibitors of mTOR. These drugs are approved for use in anticancer therapies, rejection prophylaxis after organ transplant, drug-eluting coronary stents, and the treatment of lymphangioleiomyomatosis and tuberous sclerosis.
The Uses of Rapamycin
Rapamycin is a triene macrolide discovered in 1974 as a metabolite of Streptomyces hygroscopicus found in a soil obtained on Rapa Nui (Easter Island). Rapamycin displayed potent and selective antifungal activity, notably against Candida albicans. Interest in the metabolite waned until the structural relationship to the potent immunosuppressant fujimycin (Antibiotic FK506) was recognised in the mid-1980s. This recognition led to the re-discovery of rapamycin as a highly selective antitumor and immunosuppressant. Rapamycin inhibits the activity of the protein, mTOR (mammalian target of rapamycin) which functions in a signalling pathway to promote tumor growth. Rapamycin binds to a receptor protein (FKBP12). The rapamycin/FKB12 complex then binds to mTOR and prevents interaction of mTOR with target proteins in this signalling pathway.
The Uses of Rapamycin
Labelled Rapamycin. A triene macrolide antibiotic isolated from Streptomyces hygroscopicus. Name derived from the native word for Easter Island, Rapa?Nui. Used as an immunosuppressant; antirestenotic. This compound contains aproximately 2% d0.;Labeled Sir
The Uses of Rapamycin
Rapamycin is an immunosuppressant that is used primarily to prevent the rejection of organ and bone marrow transplant. It was first described as a potent inhibitor of IL-2 activation of lymphocytes (IC50 = 5 pM). It is now known that rapamycin specifically interacts with the cytosolic FK-binding protein 12 (FKBP12) to form a complex which inhibits the mammalian target of rapamycin (mTOR) pathway by directly binding to mTOR Complex 1 (mTORC1). Rapamycin and other inhibitors of mTORC1 signaling show potential in treating cancer, adipogenesis, diabetes, tuberous sclerosis, and cardiovascular disease.[Cayman Chemical]
Background
Sirolimus, also known as rapamycin, is a macrocyclic lactone antibiotic produced by bacteria Streptomyces hygroscopicus, which was isolated from the soil of the Vai Atari region of Rapa Nui (Easter Island). It was first isolated and identified as an antifungal agent with potent anticandida activity; however, after its potent antitumor and immunosuppressive activities were later discovered, it was extensively investigated as an immunosuppressive and antitumour agent. Its primary mechanism of action is the inhibition of the mammalian target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that regulates cell growth, proliferation, and survival. mTOR is an important therapeutic target for various diseases, as it was shown to regulate longevity and maintain normal glucose homeostasis. Targeting mTOR received more attention especially in cancer, as mTOR signalling pathways are constitutively activated in many types of human cancer.
Sirolimus was first approved by the FDA in 1999 for the prophylaxis of organ rejection in patients aged 13 years and older receiving renal transplants. In November 2000, the drug was recognized by the European Agency as an alternative to calcineurin antagonists for maintenance therapy with corticosteroids. In May 2015, the FDA approved sirolimus for the treatment of patients with lymphangioleiomyomatosis. In November 2021, albumin-bound sirolimus for intravenous injection was approved by the FDA for the treatment of adults with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumour (PEComa). Sirolimus was also investigated in other cancers such as skin cancer, Kaposi’s Sarcoma, cutaneous T-cell lymphomas, and tuberous sclerosis. The topical formulation of sirolimus, marketed as HYFTOR, was approved by the FDA in April 2022: this marks the first topical treatment approved in the US for facial angiofibroma associated with tuberous sclerosis complex.
Indications
Sirolimus is indicated for the prophylaxis of organ rejection in patients aged 13 years or older receiving renal transplants. In patients at low-to moderate-immunologic risk, it is recommended that sirolimus be used initially in a regimen with cyclosporine and corticosteroids; cyclosporine should be withdrawn two to four months after transplantation. In patients at high-immunologic risk (defined as Black recipients and/or repeat renal transplant recipients who lost a previous allograft for immunologic reason and/or patients with high panel-reactive antibodies [PRA; peak PRA level > 80%]), it is recommended that sirolimus be used in combination with cyclosporine and corticosteroids for the first year following transplantation.
It is also used to treat lymphangioleiomyomatosis.
In the US, albumin-bound sirolimus for intravenous injection is indicated for the treatment of adult patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumour (PEComa).
In Europe, it is recommended that sirolimus for the prophylaxis of organ rejection in renal transplants is used in combination with cyclosporin microemulsion and corticosteroids for two to three months. Sirolimus may be continued as maintenance therapy with corticosteroids only if cyclosporin microemulsion can be progressively discontinued.
Topical sirolimus is indicated for the treatment of facial angiofibroma associated with tuberous sclerosis in adults and pediatric patients six years of age and older.
Definition
ChEBI: A macrolide isolated from Streptomyces hygroscopicus consisting of a 29-membered ring containing 4 trans double bonds, three of which are conjugated. It is an antibiotic, immunosupressive and antineoplastic agent.
Indications
Mechanistic target of rapamycin (mTOR) is a serine/threonine-specific protein kinase in the PI3/PI4-kinase family. mTOR was named after the natural macrolide rapamycin, also known as sirolimus, which was isolated from a soil sample from Easter Island in the 1970s and later evaluated as an immunosuppressive agent. The anticancer activity of rapamycin was discovered in the 1980s, although the mechanismof action and the identification of the rapamycin target, mTOR, were not elucidated until the 1990s. Rapamycin and its macrocyclic analogues, such as temsirolimus (Torisel(R), Wyeth/Pfizer) and everolimus (Afinitor(R), Novartis), are grouped as “rapalogs” that constitute the first-generation mTOR inhibitors.
Rapamycin was approved by the US FDA in 1999 as an immunosuppressive agent to prevent organ rejection in patients receiving kidney transplants. Although a large number of clinical studies have been performed to evaluate the anticancer activities of sirolimus in different types of cancers, such as invasive bladder cancer, breast cancer, and leukemia, most studies show limited efficacy. Outside oncological indications, sirolimus was approved by FDA for the treatment of a rare progressive lung disease lymphangioleiomyomatosis in 2015. Temsirolimus was approved for the treatment of advanced RCC. Everolimus was approved in the EU for the prevention of organ rejection in heart and kidney transplant recipients before FDA approved it in 2009 for the treatment of advanced RCC resistant to sunitinib or sorafenib and for the treatment of advanced or metastatic gastrointestinal and lung tumors in 2016. Additionally, rapamycin and rapalogs are being investigated as antiaging therapeutics or for the treatment of age-related diseases. Studies have revealed that mTOR activity can be retained under hypoxic conditions via mutations in the PI3K pathway, leading to increased translation and hypoxic gene expression and tumor progression.
Indications
Sirolimus (Rapamune) is structurally related to tacrolimus. It is approved for use as an adjunctive agent in combination with cyclosporine for prevention of acute renal allograft rejection. It blocks IL-2-dependent T-cell proliferation by inhibiting a cytoplasmic serine– threonine kinase. This mechanism of action is different from those of tacrolimus and cyclosporine. This allows sirolimus to augment the immunosuppressive effects of these drugs.
Manufacturing Process
Streptomyces hygroscopicus NRRL 5491 was grown and maintained on
oatmeal-tomato paste agar slants (T. G. Pridham et al.; Antibiotic Annual
1956-1957, Medical Encyclopedia Inc., New York, p. 947) and in Roux bottles
containing the same medium. Good growth was obtained after 7 days of
incubation at 28°C. Spores from one Roux bottle were washed off and
suspended into 50 ml of sterile distilled water. This suspension was used to
inoculate the first stage inoculum.The first-stage inoculum medium consisted of Emerson broth [R. L. Emerson
et al., J. Bacteriol, 52, 357 (1946)] 0.4% peptone, 0.4% sodium chloride,
0.25% yeast extract and 1% glucose; pH 7.0; flasks containing the above
medium were inoculated with 1 % of the spore suspension described above.
The inoculated flasks were incubated for 30 hours at 28°C on a reciprocating
shaker set at 65 r.p.m. (4 inch stroke).
Production stage
The production stage was run in 250-liter New Brunswick fermenters Model F-
250, equipped with automatic antifoam addition system and pH recordercontroller.
The fermenters were charged with 160 L of an aqueous production
medium consisting of the following constituents: 1.0% soluble starch; 0.5%
(NH4)2SO4; 0.5% K2HPO4; 1.5% glucose (Cerelose); 0.025% MgSO4; 0.005%
ZnSO4; 0.001% MnSO4; 0.002% FeSO47H2O; 0.2% CaCO3; 0.5% "Blackstrap"
molasses; 0.5% hydrolyzed casein; 0.2% lard oil; pH 7.1 to 7.3 of first stage
inoculum. Incubation temperature: 28°C; aeration: 0.5 vol/vol/min; agitation:
250 r.p.m. The fermenters were sterilized at 121°C for 45 min, cooled and
inoculated with one flask inoculum).
A titre of ca. 20 μg/ml, determined by microbiological assay on agar plates
seeded with Candida albicans, was reached in 5 days. The fermentation was
stopped. The fermentation mixture was extracted twice with 1 v/v of nbutanol.
The combined butanol extracts were washed with 1 v/v of water,
dried with anhydrous sodium sulfate and evaporated to dryness under reduced
pressure to yield a residue. The oily residue was extracted 3 times with 2 L of
methanol. The combined methanol extracts were passed through
diatomaceous earth (Celite) and evaporated to dryness to yield an oily residue
containing crude Rapamycin.
brand name
Rapamune (Wyeth).
Therapeutic Function
Immunosuppressive, Antifungal
General Description
Rapamycin is an anti-fungal antibiotic isolated from Streptomyces hygroscopicus. This antibiotic is active against all strains of Candida albicans. It blocks the signal transduction pathways required for the activation of T-helper cells.
Biological Activity
Antifungal and immunosuppressant. Specific inhibitor of mTOR (mammalian target of Rapamycin). Complexes with FKBP-12 and binds mTOR inhibiting its activity. Inhibits interleukin-2-induced phosphorylation and activation of p70 S6 kinase.
Biochem/physiol Actions
Rapamycin is a macrocyclic triene antibiotic possessing potent immunosuppressant and anticancer activity. It forms a complex with FKBP12 that binds to and inhibits the molecular target of rapamycin (mTOR). mTOR is a member of the phosphoinositide kinase-related kinase (PIKK) family that enhances cellular proliferation via the phosphoinositol 3-kinase/Akt signaling pathway. Inhibition of this pathway by rapamycin blocks downstream elements that result in cell cycle arrest in G1. The effectors of mTOR action include 4EBP1 and S6K1.
Pharmacokinetics
Sirolimus is an immunosuppressant drug with antifungal and antitumour effects. In animal models, sirolimus prolonged allograft survival following various organ transplants and reversed an acute rejection of heart and kidney allografts in rats. Upon oral administration of 2 mg/day and 5 mg/day, sirolimus significantly reduced the incidence of organ rejection in low- to moderate-immunologic risk renal transplant patients at six months following transplantation compared with either azathioprine or placebo. In some studies, the immunosuppressive effect of sirolimus lasted up to six months after discontinuation of therapy: this tolerization effect is alloantigen-specific. Sirolimus potently inhibits antigen-induced proliferation of T cells, B cells, and antibody production.
In rodent models of autoimmune disease, sirolimus suppressed immune-mediated events associated with systemic lupus erythematosus, collagen-induced arthritis, autoimmune type I diabetes, autoimmune myocarditis, experimental allergic encephalomyelitis, graft-versus-host disease, and autoimmune uveoretinitis.
Clinical Use
Immunosuppressant:
Prophylaxis of transplant allograft rejection
in vitro
Rapamycin (12.5-100 nM; 24 hours) treatment exerts modest inhibitory effect on lung cancer cell proliferation in a dose-dependent manner in all cell lines (A549, SPC-A-1, 95D and NCI-H446 cells) tested, achieving about 30-40% reduction in cell proliferation at 100 nM vs. ~10% reduction at 12.5 nM.
Lung cancer cell line 95D cells are exposed to Rapamycin (10 nM, 20 nM) and RP-56976 (1 nM, 10 nM) alone or in combination (Rapamycin 20 nM+ RP-56976 10 nM). After 24 hours exposure to Rapamycin or RP-56976 alone does not significantly alter the level of expression or phosphorylation of ERK1/2, whereas cells treated with the combination of Rapamycin with RP-56976 exhibit a marked reduction in the phosphorylation levels of ERK1/2.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration increased by
clarithromycin - avoid; concentration of both drugs
increased with erythromycin; concentration reduced
by rifampicin and rifabutin - avoid.
Antifungals: concentration increased by itraconazole,
fluconazole, ketoconazole, micafungin, miconazole,posaconazole and voriconazole - avoid with
itraconazole, ketoconazole and voriconazole.
Antivirals: concentration possibly increased by
atazanavir, boceprevir and lopinavir; concentration
of both drugs increased with telaprevir, reduce
dose of sirolimus; concomitant use with dasabuvir
plus ombitasvir/paritaprevir/ritonavir is not
recommended unless the benefits outweigh the risks,
if used together administer sirolimus 0.2 mg twice a
week (every 3 or 4 days on the same two days each
week). Sirolimus blood concentrations should be
monitored every 4-7 days until 3 consecutive trough
levels have shown stable concentrations of sirolimus.
Sirolimus dose and/or dosing frequency should be
adjusted as needed.
Calcium-channel blockers: concentration increased
by diltiazem; concentration of both drugs increased
with verapamil.
Ciclosporin: increased absorption of sirolimus -
give sirolimus 4 hours after ciclosporin; sirolimus
concentration increased; long-term concomitant
administration may be associated with deterioration
in renal function.
Cytotoxics: use crizotinib with caution.
Grapefruit juice: concentration of sirolimus increased
- avoid.
Mycophenolate: concomitant use of mycophenolate
and sirolimus increases plasma levels of both
sirolimus and mycophenolic acid.
Metabolism
Sirolimus undergoes extensive metabolism in the intestinal wall and liver. Sirolimus is primarily metabolized by O-demethylation and/or hydroxylation via CYP3A4 to form seven major metabolites, including hydroxy, demethyl, and hydroxydemethyl metabolites, which are pharmacologically inactive. Sirolimus also undergoes counter-transport from enterocytes of the small intestine into the gut lumen.
Metabolism
Sirolimus is metabolised by the cytochrome P450 isoenzyme CYP3A4. Metabolism occurs by demethylation or hydroxylation, and the majority of a dose is excreted via the faeces.
Storage
-20°C
References
1) Kay et al. (1991) Inhibition of T and B lymphocyte proliferation by rapamycin. Immunology, 72 544 2) Mita et al., (2003) The molecular target of rapamycin (mTOR) as a therapeutic target against cancer; Cancer Biol. Ther., 2(4 Suppl 1) S169 3) Lamming et al. ( 2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity; Science, 335 1638 4) Sarkar et al. (2009), Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies; Cell Death and Differ., 16 46
Properties of Rapamycin
| Melting point: | 183-185°C |
| Boiling point: | 799.83°C (rough estimate) |
| alpha | D25 -58.2° (methanol) |
| Density | 1.0352 (rough estimate) |
| vapor pressure | 0.56 hPa ( 20 °C) |
| refractive index | 1.5280 (estimate) |
| Flash point: | 87 °C |
| storage temp. | -20°C |
| solubility | ethanol: soluble2MM |
| pka | 10.40±0.70(Predicted) |
| form | solution |
| color | colorless to yellow |
| Water Solubility | Soluble in DMSO at 50mg/ml or methanol at 25mg/mlSoluble in alcohol, dimethyl sulfoxide and dimethyl formamide. Insoluble in water. |
| Sensitive | Moisture Sensitive/Light Sensitive/Hygroscopic |
| Merck | 14,8114 |
| Stability: | Stable for 2 years? from date of purchase as supplied.? Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
| CAS DataBase Reference | 53123-88-9(CAS DataBase Reference) |
Safety information for Rapamycin
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Rapamycin
Rapamycin manufacturer
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Rapamycin CAS 53123-88-9View Details
Rapamycin CAS 53123-88-9View Details
53123-88-9 -
 Rapamycin, from streptomyces hygroscopicus CAS 53123-88-9View Details
Rapamycin, from streptomyces hygroscopicus CAS 53123-88-9View Details
53123-88-9 -
 Rapamycin, ≥98% (HPLC) CAS 53123-88-9View Details
Rapamycin, ≥98% (HPLC) CAS 53123-88-9View Details
53123-88-9 -
 Rapamycin 95.00% CAS 53123-88-9View Details
Rapamycin 95.00% CAS 53123-88-9View Details
53123-88-9 -
 Rapamycin CASView Details
Rapamycin CASView Details -
 Rapamycin CASView Details
Rapamycin CASView Details -
 Sirolimus Raw Material, Grade Standard: Bio-Tech Grade, 53123-88-9View Details
Sirolimus Raw Material, Grade Standard: Bio-Tech Grade, 53123-88-9View Details
53123-88-9 -
 Rapamycin CAS 53123-88-9View Details
Rapamycin CAS 53123-88-9View Details
53123-88-9
