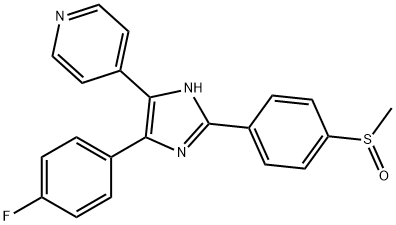
SB 203580
Synonym(s):4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole;4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole, p38 MAP Kinase Inhibitor XVI;SB 203580 - CAS 152121-47-6 - Calbiochem
- CAS NO.:152121-47-6
- Empirical Formula: C21H16FN3OS
- Molecular Weight: 377.43
- MDL number: MFCD00922198
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 21:14:07
What is SB 203580?
Description
SB 203580 (152121-47-6) is a potent and selective inhibitor of p38 MAP kinase, IC50=50 and 500 nM for p38 and p38β2 respectively.1 No other kinases (in a panel of 30) were significantly inhibited including p38γ and δ at 10 μM.2?A potent inhibitor of inflammatory cytokine production (IC50=15-25 mg/kg in mice and rats) in animal models of arthritis, bone resorption, endotoxin shock and immune function.3
Chemical properties
White to Off-White Solid
The Uses of SB 203580
MDCK cells were treated with SB 203580 to study the role of MAPK signaling in inducing hypertonicity of cell membrane and potassium depletion.16 It was used to inhibit MAPK signaling in mouse cortical neurons17 and human hepatocellular carcinoma cell lines.18
The Uses of SB 203580
A pyridinyl imidazole which acts as a specific inhibitor of p38 MAP kinase. Does not inhibit the MAP kinase homologs JNK and p42 MAP kinase
The Uses of SB 203580
A p38 MAPK inhibitor with an IC50 range of 0.3-0.5 μM and blocks PKB phosphorylation with an IC50 range of 3-5 μM.
What are the applications of Application
SB 203580 is a pyridinyl imidazole and specific inhibitor that suppresses p38 mediated activation of MK2.
Definition
ChEBI: A member of the class of imidazoles carrying 4-methylsulfinylphenyl, 4-pyridyl and 4-fluorophenyl substituents at positions 2, 4 and 5 respectively. An inhibitor of mitogen-activated protein kinase.
General Description
Reduces epirubicin-induced cell injury and caspase-3/7 activity. A highly specific, potent, cell-permeable, selective, reversible, and ATP-competitive inhibitor of p38 kinase (IC50 = 34 nM in vitro, 600 nM in cells). Also known as reactivating kinase (RK) and CSBP (cytokine synthesis anti-inflammatory drug binding protein). Does not significantly inhibit JNK or p42 MAP kinase even at 100 μM. Inhibits IL-1 and TNF-α production from LPS-stimulated human monocytes and the human monocyte cell line THP-1 (IC50 = 50-100 nM). SB 203580 has also been shown to be an effective inhibitor of inflammatory cytokine production in vivo in both mice and rats.
Biological Activity
Water-soluble salt of SB 203580 (4-[5-(4-Fluorophenyl)-2-[4-(methylsulfonyl)phenyl]-1H-imidazol-4-yl]pyridine ). Selective inhibitor of p38 mitogen-activated protein kinase (IC 50 values are 50 and 500 nM for SAPK2a/p38 and SAPK2b/p38 β 2 respectively). Displays 100-500-fold selectivity over LCK, GSK3 β and PKB α . Shown to inhibit interleukin-2-induced T-cell proliferation, cyclooxygenase-1 and -2, and thromboxane synthase.
Biochem/physiol Actions
SB 203580 is a pyridinyl imidazole that suppresses the activation of MAPKAP kinase-2 and inhibits the phosphorylation of heat shock protein (HSP) 27 in response to IL-1, cellular stresses and bacterial endotoxin in vivo. It does not inhibit JNK or p42 MAP kinase and therefore, is useful for studying the physiological roles and targets of p38 MAPK and MAPKAP kinase-2. It has been shown to induce the activation of the serine/threonine kinase Raf-1 and has been reported to inhibit cytokine production.
storage
+4°C (desiccate)
References
1) Cuenda et al. (1995), SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1; FEBS Lett., 364 229 2) Davies et al. (2000), Specificity and mechanism of action of some commonly used protein kinase inhibitors; Biochem. J., 351 95 3) Badger et al. (1996), Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function; Pharmacol. Exp. Ther. 279 1453
Properties of SB 203580
| Melting point: | 144-146 |
| Boiling point: | 615.6±55.0 °C(Predicted) |
| Density | 1.4 |
| storage temp. | -20°C |
| solubility | DMSO: 50 mg/mL |
| form | solid |
| pka | 9.60±0.10(Predicted) |
| color | white to off-white |
| Stability: | -200C |
| CAS DataBase Reference | 152121-47-6(CAS DataBase Reference) |
Safety information for SB 203580
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
Computed Descriptors for SB 203580
| InChIKey | CDMGBJANTYXAIV-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![2-(5-Benzo[1,3]dioxol-5-yl-2-tert-butyl-3H-imidazol-4-yl)-6-methylpyridine hydrate hydrochloride](https://img.chemicalbook.in/CAS/GIF/694433-59-5.gif)
You may like
-
 SB 203580 CAS 152121-47-6View Details
SB 203580 CAS 152121-47-6View Details
152121-47-6 -
 SB 203580, ≥98% (HPLC) CAS 152121-47-6View Details
SB 203580, ≥98% (HPLC) CAS 152121-47-6View Details
152121-47-6 -
 SB203580 >95% CAS 152121-47-6View Details
SB203580 >95% CAS 152121-47-6View Details
152121-47-6 -
 SB 203580 CAS 152121-47-6View Details
SB 203580 CAS 152121-47-6View Details
152121-47-6 -
 SB 203580 CAS 152121-47-6View Details
SB 203580 CAS 152121-47-6View Details
152121-47-6 -
 SB 203580 CASView Details
SB 203580 CASView Details -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1