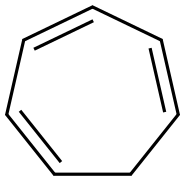
Tropolone
Synonym(s):2-Hydroxy-2,4,6-cycloheptatrien-1-one;Tropolone
- CAS NO.:533-75-5
- Empirical Formula: C7H6O2
- Molecular Weight: 122.12
- MDL number: MFCD00004158
- EINECS: 208-577-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
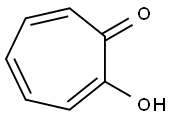
What is Tropolone?
Chemical properties
White to light yellow crystalline
The Uses of Tropolone
Tropolone is a sensitive reagent for reducing sugars. A non-benzenoid aromatic compound, Reagent for the preparation of fused heterocycles and complexes of Ga(III) and In(III).
The Uses of Tropolone
Reagent for the preparation of fused heterocycles1 and complexes of Ga(III) and In(III).2 Used as medicine and dye intermediates.
What are the applications of Application
Tropolone is a reagent for the preparation of fused heterocycles and complexes of Ga(III) and In(III)
Definition
ChEBI: Tropolone is a cyclic ketone that is cyclohepta-2,4,6-trien-1-one substituted by a hydroxy group at position 2. It is a toxin produced by the agricultural pathogen Burkholderia plantarii. It has a role as a bacterial metabolite, a toxin and a fungicide. It is a cyclic ketone, an enol and an alpha-hydroxy ketone. It derives from a hydride of a cyclohepta-1,3,5-triene.
Flammability and Explosibility
Not classified
Synthesis
To a mixture of sodium hydroxide (33 g) with glacial acetic acid (270 ml) was added dropwise via funnel 7,7-dichlorobicyclo[3,2,0]hepta-2-ene-6-ketone (33.5 g) under nitrogen, and the mixture was heated to reflux for 8 hours. After PH was adjusted to 1 with hydrochloric acid (50 ml), the mixture was filtered and extracted with benzene (3 × 90ml). The combined extracts were concentrated in vacuo to give brownish black oil.Fraction of reduced pressure distillation at 100 °C/67 Pa was collected to give gross product as a pale yellow solid. Recrystallization from mixture of dichloromethane and pentane (1/4, V/V) gave analytically pure product Tropolone(17.8 g,77%) as a white needle crystal, m.p. 50~51 °C.
Purification Methods
Crystallise tropolone from hexane or pet ether and sublime it at 40o/4mm. Also distil it at high vacuum. [Beilstein 8 IV 159.]
Properties of Tropolone
| Melting point: | 50-52 °C (lit.) |
| Boiling point: | 80-84 °C/0.1 mmHg (lit.) |
| Density | 1.1483 (rough estimate) |
| vapor pressure | 3.4Pa at 25℃ |
| refractive index | 1.5286 (estimate) |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | 40.9g/l (experimental) |
| form | Crystalline Powder |
| pka | 6.7(at 25℃) |
| color | Off-white to cream-beige |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| BRN | 1904978 |
| CAS DataBase Reference | 533-75-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,4,6-Cycloheptatrien-1-one, 2-hydroxy-(533-75-5) |
Safety information for Tropolone
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tropolone
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Tropolone 98% (GC) CAS 533-75-5View Details
Tropolone 98% (GC) CAS 533-75-5View Details
533-75-5 -
 Tropolone CAS 533-75-5View Details
Tropolone CAS 533-75-5View Details
533-75-5 -
 Tropolone, 98% CAS 533-75-5View Details
Tropolone, 98% CAS 533-75-5View Details
533-75-5 -
 Tropolone 98% CAS 533-75-5View Details
Tropolone 98% CAS 533-75-5View Details
533-75-5 -
 Tropolone CAS 533-75-5View Details
Tropolone CAS 533-75-5View Details
533-75-5 -
 Tropolone CAS 533-75-5View Details
Tropolone CAS 533-75-5View Details
533-75-5 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1