Cycloheptatriene
- CAS NO.:544-25-2
- Empirical Formula: C7H8
- Molecular Weight: 92.14
- MDL number: MFCD00004146
- EINECS: 208-866-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
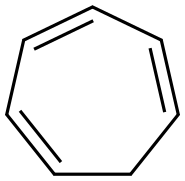
What is Cycloheptatriene?
Chemical properties
Cycloheptatriene is a clear yellow to brownish liquid. Insoluble in water and less dense than water. Vapors heavier than air. Inhalation of high concentrations may have a narcotic effect. It is a ligand in organometallic chemistry and as a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge with respect to the other atoms.
The Uses of Cycloheptatriene
1,3,5-Cycloheptatriene functions as a ligand in organometallic chemistry and also as a building block in organic synthetic processes. 1,3,5-Cycloheptatriene is applied in cycloheptatrienyl transition zirconium complexes formation and in other organic complex formation processes.
Definition
ChEBI: A cycloheptatriene with unsaturation at positions 1, 3 and 5.
Synthesis Reference(s)
Journal of the American Chemical Society, 81, p. 5009, 1959 DOI: 10.1021/ja01527a075
Tetrahedron, 21, p. 1673, 1965 DOI: 10.1016/S0040-4020(01)98637-5
General Description
A colorless liquid. Insoluble in water and less dense than water. Flash point 35°F. Vapors heavier than air. Inhalation of high concentrations may have a narcotic effect. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Cycloheptatriene may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions.
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Poison by ingestion and skin contact. Mutation data reported. A very dangerous fire hazard when exposed to heat, flame, or oxidzers. Potentially violent reaction with nitrogen monoxide. Whenheated to decomposition it emits acrid smoke and fumes.
Purification Methods
Wash the triene with alkali, then fractionally distil it. Store it under N2 or Ar as it resinifies in air. [Dryden J Am Chem Soc 76 2814 1954, Kohler et al. J Am Chem Soc 61 1057 1939, Beilstein 5 IV 280.]
Properties of Cycloheptatriene
| Melting point: | -79.5°C |
| Boiling point: | 116-117 °C (lit.) |
| Density | 0.888 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 80 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethanol (Slightly) |
| form | clear liquid |
| color | Colorless to Light orange to Yellow |
| Water Solubility | insoluble |
| BRN | 506066 |
| Dielectric constant | 2.54 |
| CAS DataBase Reference | 544-25-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3,5-Cycloheptatriene(544-25-2) |
| EPA Substance Registry System | 1,3,5-Cycloheptatriene (544-25-2) |
Safety information for Cycloheptatriene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cycloheptatriene
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 1,3,5-Cycloheptatriene CAS 544-25-2View Details
1,3,5-Cycloheptatriene CAS 544-25-2View Details
544-25-2 -
 Cyclohepta-1,3,5-triene tech CAS 544-25-2View Details
Cyclohepta-1,3,5-triene tech CAS 544-25-2View Details
544-25-2 -
 Cycloheptatriene CAS 544-25-2View Details
Cycloheptatriene CAS 544-25-2View Details
544-25-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
